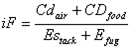Intake fractions of cadmium emissions from a large zinc smelter
- The text on this page is taken from an equivalent page of the IEHIAS-project.
Aims: to assess total intake of, and intake fractions for, cadmium emissions from a large zinc smelter, both from airborne transport and via food.
Methods: Atmospheric dispersion of cadmium emissions from the Avonmouth zinc smelter were modelled using the ADMS-Urban dispersion model, using representative meteorological data (for 2001) and concentrations estimated for all postcode centroids within 20 km of the site. Intake via inhalation was modelled using indoor penetration rates, time activity data and inhalation rates derived from a literature review. Intake via food was modelled on the basis of soil cadmium concentrations obtained from a field survey in the study area. Plant and animal uptake and transfers into food products were estimated on the basis of data from literature.
Methods
Study area and emission sources
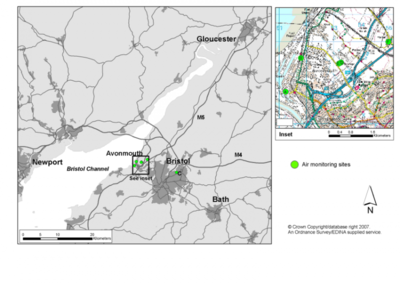
The study area was focused on the zinc smelter at Avonmouth, in south-west England. The town of Avonmouth stands on the edge of the Bristol Channel, with the industrial cities of Newport about 20km to the northwest, and the major city of Bristol (population 380,000) about 9km to the southeast. Other major settlements within the surrounding region include Bath (population 169,000) and Gloucester (110,000 population) (Figure 1). The topography is for the most part low-lying, with an altitude range within the surrounding 20 km of 308 metres. The climate is temperate oceanic, with prevailing winds from the south-west – a wind flow which is to some extent strengthened by the Bristol Channel.
Avonmouth itself has long been an important area for manufacturing and heavy industrial activity, but the Britannica Zinc Smelter was the only major source of Cd emission in the region – and until it closed in 2002, the largest single emission source in the UK (Filzek et al. 2004). The site lies in the northern quarter of Avonmouth (Figure 1), and the site comprised up to 12 operating stacks set within an area of about 60 ha.
Modelling
Emission sources and exposure pathways

Computation of intake fractions from an industrial source such as the Avonmouth zinc smelter needs to take account both of the different emission sources and dispersion pathways involved, and of the long timescales during which released Cd may remain in the environment before being absorbed by humans. As with other industrial sources, the main exposure pathways are via the air, drinking water and food. Direct releases into the environment occur largely through stack emissions into the atmosphere, primarily in aerosol form (as cadmium oxide); the smaller amounts released in gaseous form quickly become adsorbed onto particles. In addition, considerable quantities may be released as a result of fugitive emissions from the smelter, including escape from pipes and valves, and in the form of dust from stored ores, during transport and during milling. Coarser particles, including especially dusts emitted from ground-level sources, are likely to travel only short distances before removal by dry or wet deposition. On the other hand, finer particles, released in the heated plumes with high emission velocities from the main stacks, can be carried to considerable elevations in the atmosphere and laterally over distances of thousands of kilometres. Most of the cadmium in the atmosphere has thus been shown to be concentrated in finer particles (PM1 or less). As such, it lies within the respirable particle size fraction (Milacic and Dolinsek 1994, Williams and Harrison 1984).
Removal of Cd from the atmosphere occurs in a number of ways: by dry deposition, under the influence of gravity, by wet deposition and washout by rain, as a result of filtering by vegetation and other physical barriers (e.g. ventilation systems in buildings), and inhalation by animals (including humans). Over land, deposited cadmium tends to accumulate in the soil. Cd binds strongly to organic matter and, to a lesser extent, to clays and is thus somewhat immobile, especially in humus-rich or fine-grained soils. Residence times in the soil are therefore long, and most of the Cd accumulates in the topsoil in non-bioavailable form. Plant uptake is therefore slow, and inputs into the human food chain restricted. Leaching is also inhibited (Eriksson 1988, Li and Shuman1996), with the consequence that opportunities for Cd input into drinking waters is limited, and measured Cd concentrations in treated waters are generally low (Ryan et al. 2000). Loss by soil erosion, on the other hand, can be substantial, with the consequence that much of the Cd reaching streams is in insoluble form, attached to soil particles. Stream sediment loads tend to increase with slope angle because of the increased erosion potential (Korentajer et al. 1993).
Based on these pathways, the intake of cadmium from the Avonmouth zinc smelter may be summarised as in Figure 2. As this indicates, the intake pathway via drinking water is considered to be negligible, and is ignored here. Intake fractions are thus estimated only for the air and food pathways. The former may be assumed to occur mainly in the near vicinity of the smelter (though long distance transport of small amounts of Cd must also be acknowledged); the latter affects local populations through consumption of locally-grown foodstuffs but also spreads more widely via commercial food distribution systems. It should also be noted that numerous secondary intake pathways also exist, for example from solid wastes and sewage, or re-entrainment of dust and sediments. These are not allowed for in the assessment, but their implications are discussed below.
Emissions
The zinc smelter at Avonmouth was operational between 1920 and 2002, during which time production levels, technologies and emissions varied substantially. Under the Integrated Pollution Prevention and Control legislation, monitoring of emissions has been obligatory since the early 1990s, and data prior to that were collected by the operators. For this study, however, detailed data could only be obtained on stack emissions, and these only for the period since 1996 (subsequently, annual data for earlier years were located and made available). Over the period 1996-2002, these averaged 1235 kg/year. Although this almost certainly under-estimates past stack emissions, the pattern of dispersion is likely to have remained largely unchanged; intake as a proportion of emissions (i.e. the intake fraction) is also likely to have remained broadly constant, though some variation will have occurred because of population changes in the local area. Detailed data on fugitive emissions were not available, but were typically at least the equivalent of those via the stack (Bristol City Council 2003). For the analysis used here, therefore, both sets of emissions were set to 950 kg/year. Releases to water are considered to have been negligible.
Atmospheric dispersion
Atmospheric dispersion of the emitted Cd was modelled using the ADMS-Urban model. This is a semi-Gaussian (non-Gaussian in the vertical profile in convective conditions) dispersion model. It shows a number of improvements on previous generations of model including more advanced methods for modelling the near-surface layer and more powerful techniques for parameterising meteorological conditions (CERC 1999). It has been widely validated under laboratory and field conditions, and shown to give reliable estimates of concentrations in well-parameterised conditions (Carruthers et al. 1999, 2000). Uncertainties for mean annual concentration have been reported to be 20% or less (Colvile et al. 2002). The model includes a terrain function to take account of topographic effects on dispersion and an intelligent interpolator for generating high resolution maps of pollutant surfaces.
The ADMS-Urban model was run for the year 2001 using hourly meteorological data obtained from Filton national weather station, 8km to the east of Avonmouth. The emissions arising from the 12 operating stacks were modelled in point source mode whereas the fugitive emissions were modelled in area source mode. Table 1 shows the model inputs and assumptions. Cadmium concentrations were modelled for a 40 x 40km grid around the smelter with a 400m resolution. Model performance was investigated by comparing predicted concentrations with measured concentrations at six sites for which monitoring had been done during 2000 and 2001.
Intake from the air
Human intake of atmospheric Cd occurs through inhalation of contaminated air in a range of different environments and in different physiological circumstances. Intake rates thus depend both on where people spend their time and on their activities in those micro-environments. Detailed data on time activity were not available for the study population, so estimates were made for relevant parameters from the literature.
In this context an important distinction needs to be made between indoor and outdoor exposures. Most people spend a large proportion of their time indoors. Data from the UK Time Use Survey (National Statistics 2002), for example, show that about 50% of the day, on average, is spent at home indoors, and 30% at work or school; a further 10% of time is spent in other indoor premises, such as the houses of friends or shops. Indoor exposures of Cd released from the smelter thus depend fundamentally on its ability to penetrate buildings. Few measurements of indoor and outdoor concentrations of Cd in the UK have been undertaken, but since most Cd is in aerosol form ratios may be imputed from studies of fine particulates. In homes without major indoor sources (e.g. smoking, solid-fuel fires), these suggest that indoor concentrations are typically 50-80% of ambient concentrations, though with marked seasonal variations depending on ventilation practices (Dimitroulopoulou et al. 2001, Kingham et al. 2000, Milner et al. 2004, Wallace 1996). In buildings with air conditioning, such as offices, indoor concentrations tend to be lower, generally in the order of 30-50% of outdoor concentrations (Janssen et al. 200, Wallace 1996). Here, a mid-point ratio of 0.65 was used for homes and 0.40 for workplace environments. These two micro-environmental factors were then time-weighted, by 0.5 and 0.4 respectively, to take account of the proportion of the day spent in each micro-environment. Ambient concentrations were weighted by 0.1 to reflect time spent out of doors (Table 1).
| Parameter | Value | Source/comment |
|---|---|---|
| Stack emissions | ||
| Stack height (average 12 stacks) | 41 m | Monitoring by operators |
| Exit velocity (average 12 stacks) | 27 m/s | Monitoring by operators |
| Gas temperature (average 12 stacks) | 46 C | Monitoring by operators |
| Emission rate | 950 kg/yr | Monitoring by operators |
| Fugitive emissions | ||
| Stack height | 0 m | |
| Exit velocity | 0 m/s | |
| Gas temperature | 0 C | |
| Emission rate | 950 kg/yr | Estimate by operators |
| Dispersion conditions | ||
| Surface roughness | 0.5 | |
| Minimum Monin-Obukhov length (m) | 30 | Mixed urban/rural land |
| Indoor:outdoor ratios | ||
| Home | 0.65 | |
| Work/school | 0.4 | |
| Time activity | ||
| Proportion of spent time indoors at home | 0.5 | National Statistics (2002) |
| Proportion of time spent indoors at work | 0.4 | National Statistics (2002) |
| Proportion of time spent outdoors | 0.1 | National Statistics (2002) |
| Inhalation rates | ||
| Indoor inhalation rate: infants (< 1 year) | 0.25 m3/hr | Based on Layton (1993) |
| Indoor inhalation rates: others | 0.5 m3/hr | |
| Outdoor inhalation rate: infants (< 1 year) | 0.75 m3/hr | Based on Layton (1993) |
| Outdoor inhalation rates: others | 1.5 m3/hr | |
Physical activity levels in these different micro-environments vary. Again, detailed data are not available for the study population, but the UK Time Use Survey (2000) suggests that people spend less than 10% of their time in moderate to intense physical activities, such as heavy work or exercise-taking. Inhalation rates likewise vary, both in response to activity levels and because of differences in physiological function (e.g. associated with age, gender, body mass and physical health). Estimates of inhalation rates reported in the literature show a considerable range (e.g. US Environmental Protection Agency 1996, Layton 1993, Marshall et al. 2005), but in the UK an average value of 0.85 m3/hour is widely used for dose estimates for adults, while Layton (1993) has suggested rates of 0.2 m3/hour and 0.36 m3/hour for children aged <1 year and 1-10 years, respectively. Here we assume inhalation rates for of 0.5 m3/hr and 1.5 m3/hr for indoors and outdoors respectively, with values of half these for children less than one year of age (Table 1).
Intake from food
As noted, Cd enters foodstuffs largely as a result of uptake by plants growing in Cd-contaminated soils. Soil contamination from the smelter is likely to occur mainly as a result of wet and dry deposition from the atmosphere, and might thus be expected broadly to reflect atmospheric concentrations in the surrounding area. Measurements of soil Cd were, in fact, made during the 1980s and 1990s as part of a study by Long Ashton Research Station but data on Cd uptake by crops or animals in the area are not available. Data have been collected on consumption of locally grown vegetables, and these along with locally produced meat and dairy products may be a significant exposure source (Hellström et al. 2007). Nevertheless, it cannot be assumed that intake via food is restricted to the local population; instead, agricultural produce is likely to find its way to a large and widely dispersed population, via commercial food distribution.
Cd intake via food was therefore estimated at the general population level by modelling transfers from soils into foodstuffs. The first step in this sequence involves uptake by growing crops. As many studies have indicated, the processes involved are complex and Cd concentrations vary substantially between different crops, management regimes and soils – so much so that Olsson et al. (2005) conclude that “There is no known means at present of predicting Cd uptake by crops in a specific field with any degree of accuracy”. Higher rates of uptake, for example, tend to be seen for vegetables and salad crops (e.g. spinach, carrots, lettuce, radishes), with lower rates for cereals and grasses (Davis 1984, Olsson et al. 2005, (Mueller and Anke 1994, van Driel and Smilde 1990, van Lune and Zwart 1997). Cd mobility in the soil is also strongly influenced by cation exchange capacity, so uptake rates tend to be reduced with higher pH, organic matter content and clay content (Eriksson 1988, Gray et al. 1999a, 1999b, Guttormsen et al. 1995, He and Singh 1994). Liming may likewise reduce Cd uptake (Eriksson 1988, Hatch et al. 1988, McBride 2002, Li and Shuman1996). In addition, factors such as fertiliser practice, harvesting regime and crop rotations may affect uptake rates (Davis 1984).
Although a general relationship is commonly observed between Cd concentrations in the soil and in crops, therefore, the slope, strength and shape of the association seems to vary to a considerable degree. While some studies have suggested a linear relationship (Jackson and Alloway 1991, Guttormsen et al. 1995, He and Singh 1994, Hooda et al. 1997), others have reported non-linear relationships, characterised by logarithmic or power models (Davis 1984, Dudka et al. 1998, Garrett et al. 1998, Gerritse et al. 1983, Hooda et al. 1997, Krauss et al. 2002). These differences may reflect a number of factors, including methodological variables such as the sampling and extraction medium, as well as soil or management conditions. Some of the linear associations seen in some studies may be a reflection of the limited range of soil Cd concentration studied; inspection of some of the reported data also suggests that non-linear models might fit the data at least as well. Overall, a convex association seems most plausible, on the basis that plant uptake of Cd will be inhibited to some degree at higher concentrations. Krauss et al. (2002) thus suggest that Freundlich-type equations, of the form Y = a Ln(X) – ln(b), provide a general characterisation of the associations.
For the analysis here, data on agricultural land use in the study area were obtained from the June Agricultural Returns, which report crop areas and livestock numbers for each farm in the UK (aggregated to ward level). Crops were classified into seven broad groups: potatoes, other root crops, green vegetables, wheat, barley, other cereals and grass and mapped at ward level. Average annual production rates (tonnes/ha) were estimated for each group based on national statistics (DEFRA 2004, 2006) and these used to determine the total annual biomass production for each crop group within each ward; for grass yields recent data were not available, so estimates were made by the authors based on a range of study data. Uptake functions for each of the crop groups were then derived from the literature (Table 2). All functions used relate to total soil Cd.
The soil Cd and pH concentrations were then interpolated using inverse distance weighting, in ArcGIS, and averages calculated for each ward. Measured data on clay content (required for the assessment of uptake by wheat, root vegetables and green vegetables) were not available, but inspection of soil survey records for the study area suggest that most of the soils are loams or clay-loams, in which the clay content is likely to range between about 15 and 30%. An average of 20% was imputed here.
Total Cd uptake by each crop in each ward was then assessed as follows:
where:
Cdcrop is the total Cd uptake (kg in edible dry matter),
![]() is the average Cd concentration in the crop (ug/g edible dry biomass) calculated from the relevant equation in Table 2
is the average Cd concentration in the crop (ug/g edible dry biomass) calculated from the relevant equation in Table 2
A is the planted area of the crop (ha)
Y is the average crop yield (tonnes/ha)
i and j define the crop group and ward, respectively.
| Crop/product | Average yield (t/ha) | Source | Wastage rate (%) | Source | Plant uptake rate | Comment/Source |
|---|---|---|---|---|---|---|
| Potatoes | 44.0 | DEFRA (2006) | 25 | British Potato Council (2006) | 0.284*Soil0.599 | Dudka et al. (1996) |
| Other root vegetables | 46.4 | DEFRA (2004) | 15 | DEFRA (2004) | 3.44 + 0.284*Soil - 0.328*pH - 0.017*Clay | Hooda et al. (1997) |
| Green vegetables | 7.8 | DEFRA (2004) | 15 | DEFRA (2004) | 29.43 + 1.919*Soil - 3.93*pH - 0.087*Clay | Hooda et al. (1997) |
| Wheat (grain) | 7.4 | DEFRA (2007) | 10 (20a) | Authors’ estimates | 0.990 + 0.183*Soil - 0.156*pH - 0.003*Clay | Hooda et al. (1997) |
| Barley (grain) | 5.6 | DEFRA (2007) | 10 (20a) | Authors’ estimates | 0.188*Soil0.323 | Dudka et al. (1996) |
| Other cereals | 5.8 | DEFRA (2007) | 10 (20a) | Authors’ estimates | 0.188*Soil0.323 | Dudka et al. (1996)c |
| Grass | 7.5 | Authors’ estimates | 10b | Authors’ estimates | 0.333*Soil0.328 | Dudka et al. (1996): generalised from equations for grass and clover |
- Notes: a – losses in livestock feeding; b – field losses during grazing; c – function for barley reported by Dudka et al. applied to other cereals
In the UK, vegetables and potatoes are grown primarily for human consumption, either fresh or in processed form. Consumption will nevertheless not account for the complete harvested crop, because of losses during transport, storage and processing. Whilst all the Cd contained in the consumed edible material will be taken in, not all will be retained in the body due to losses by excretion. Estimates of wastage rates during harvesting and storage/transport are available for some crops: for potatoes a figure of 18% is quoted by the British Potato Council (2006); for other vegetables wastage rates of ca. 5% are cited (DEFRA 2004). To these must be added losses during food processing, preparation and consumption. Detailed data on these are not available, but rates of 10% of food wastage in the home have been reported (Foster et al. 2006). Total wastage rates used in the analysis are summarised in Table 2.
In the case of cereals, only a minority of the production is used for human consumption, mainly through milling for flour and malting for use in beer-making. Detailed information on the usage of cereal production in the UK is surprisingly difficult to obtain, but data collected by Lang and Allin (2006) suggest that about 37% of winter wheat and 27% of winter barley produced in 2004 was used for human consumption, while a small amount (ca. 3%) was retained for seed or left unsold; the remainder is used primarily for animal feed. These statistics were thus used as a basis for allocating cereal production in the study area. Losses of 20% were estimated between field and consumption on the basis of the limited available data.
For these groups of crops, human intake was thus estimated as follows:
where
Cdveg is the human intake of Cd (kg) from the crop
Wcrop is the wastage rate (proportional).
The remainder of the cereals (i.e. 60% of wheat and 70% for other cereals) and all the grass production was assumed to be used for animal feedstuff. As for humans, consumption will be incomplete due to wastage in the field (e.g. through trampling and rejection of grass), losses during storage and transport (e.g. spillage, pest damage), and wastage during processing. Again, data on these losses are not well recorded, but rates of at least 20% seem feasible.
Not all the Cd taken in by animals is retained within the body; a large proportion is likely to be excreted. Some, also, will be stored in non-edible parts of the animal. Only a relatively small proportion of the total intake thus accumulates in the meat or milk and becomes available for human consumption. As in humans, Cd targets specific organs, especially the kidney and liver, and accumulation in other parts of the body is limited. The main potential for human exposures is thus likely to be through consumption of visceral organs (Sharma and Sharma 1979).
Data on rates of retention are limited, and general relationships between Cd concentration in grass or other feedstuffs and in edible parts of the animal seem not to have been developed. While several studies have suggested that Cd retention in animals is negligible (Sharma et al. 1979, van Bruwaene et al. 1984), others have shown increased concentrations in milk and meat in animals feeding on Cd-enriched fodder (Olsson et al. 2005, Vidovic et al. 2005). Johnson et al. (1981) reported a retention rate of 0.09% for cadmium given to cattle in animal feed, while Spadaro and Rabl (2004) used biotransferability functions of 0.00012 for meat and 0.0000065 for milk as part of a pathway analysis of potential health effects of Cd. Here, therefore, a simple proportional function of 0.0001 was applied, on the assumption that no more than 0.1% of the Cd intake is retained in edible animal products. Again, further losses also have to be allowed for between slaughter and consumption, and a wastage rate of 20% was assumed. In addition, not all livestock end up being consumed: the June Agricultural Returns for the study area, it was suggest that only about 50% of livestock enter the human food chain.
On this basis, human intake of Cd via animal products was estimated as:
where
Cdstockis the human Cd intake from livestock products
Rstock is the retention rate of Cd in the livestock (assumed to be 0.0001)
Wstock is the wastage rate between animal and human consumption (assumed to be 0.2)
Hstock is the proportion of livestock used for human consumption (estimated as 0.5).
Total intake from food was then computed as the sum of the direct intake from crops and that from livestock products:
Intake fraction
The intake fraction represents the ratio of total intake and total emissions within a defined (and linked) period of time. In the case of airborne Cd, intake occurs within a short interval of release; averaging over a period of one year thus provides an adequate basis for assessment. For intake via food, latencies may be much longer (potentially many decades) due to long-term retention in soils, plants, livestock and stored food products. In these cases, intake fractions should be assessed over longer time periods (or over the full life-cycle from initial operation of the site until eventual decay to background concentrations of soil concentrations). In this analysis a life-cycle assessment was not possible, due to lack of historic data, not only on emissions but also other system conditions, such as soil concentrations and land use. On the other hand, an approximation can be made under conditions of steady state equilibrium – i.e. when annual inputs to soils are roughly matched by plant removal and losses to leaching, erosion and runoff. Steady state was accordingly assumed for this analysis, and annual intake via both the atmosphere and food was related to the annual emission rate from the stack and fugitive sources:
References
- BristolCity Council June 2003 Environment Act 1995. Local air quality management updating and screening assessment.]
- British Potato Council 2006 [ www.potato.org.uk Yearbook of potato statistics in Great Britain.] May 2006 edition. Market Information and Statistics Department
- Carruthers, D.J., Edmunds, H.A., Lester, A.E., McHugh, C.A. and Singles, R.J. 2000 Use and validation of ADMS-Urban in contrasting urban and industrial locations. International Journal of Environment and Pollution 14, 364-374.
- Carruthers, D.J., Mckeown, A.M., Hall, D.J. and Porter, S. 1999 Validation of ADMS against wind tunnel data of dispersion from chemical warehouse fires. Atmospheric Environment 331937-1953.
- CERC Ltd, 1999ADMS-Urban. An urban air quality management system. User guide. Cambridge: CERC Ltd
- Colvile, R.N., Woodfield, N.K., Carruthers, D.J., Fisher, B.E.A., Rickard, A., Neville, S. and Hughes, A. 2002 Uncertainty in dispersion modelling and urban air quality mapping. Environmental Science and Policy5, 207–220.
- Davis, R.D. 1984 Cadmium – a complex environmental problem. Part II. Cadmium in sludges used as fertilizer. Experientia 40, 117-126.
- DEFRA 2004 [www.defra.gov.uk/ Monthly report on the vegetable crops in England and Wales.] Position as of 31 March 2004.
- DEFRA 2006 Agriculture in the UK 2006 –Tables and charts.
- Dimitroulopoulou, C., Ashmore, M.R., Byrne, M.A. and Kinnersley, R.P. 2001 Modelling of indoor exposure to nitrogen dioxide in the UK. Atmospheric Environment 35, 269-279.
- Dudka, S., Piotrowska, M. and Terelak, H. 1996 Transfer of cadmium, lead, and zinc from industrially contaminated soil to crop plants: a field study. Environmental Pollution94, 181-188.
- Eriksson, J.R 1988 The effects of clay, organic matter and time on adsorption and plant uptake of cadmium added to the soil. Water, Air, and Soil Pollution 40, 359-373.
- Filzek, P. B, Spurgeon D.J, Broll G, Svendsen C, Hankard P.K, Kammenga J.E, Donker M.H. and Weeks J.M. 2004 Pedological characterisation of sites along a transect from a primary Cadmium/Lead/Zinc smelting works. Ecotoxicology, 13(8), 725-737
- Foster, C., Green, K., Bleda, M., Dewick, P., Evans, B., Flynn, A. and Mylan, M. 2006 Environmental impacts of food production and consumption: a report to the Department for Environment, Food and Rural Affairs. Manchester: Manchester Business School.
- Garrett, R.G., MacLaurin, A.I., Gawalko, E.J, Tkachuk, R. and Hall, G.E.M. 1998 A prediction model for estimating the cadmium content of durum wheat from soil chemistry. Journal of Geochemical Exploration64, 101–110.
- Gerritse, R.G., van Driel, W., Smilde, K.W. and van Luit, B. 1983 Uptake of heavy metals by crops in relation to their concentration in the soil solution. Plant and Soil75, 393-404.
- Gray, C.W., McLaren, R.G., Roberts, A.H.C. and Condron, L.M. 1999a Effect of soil pH on cadmium phytoavailability in some New Zealand soils. New ZealandJournal of Crop and Horticultural Science, 1999, Vol.27: 169-179.
- Gray, C.W., McLaren, R.G., Roberts, A.H.C, Condron, L.M. 1999b Solubility, sorption and desorption of native and added cadmium in relation to properties of soils in New Zealand. European Journal of Soil Science 50: 127-13 7.
- Guttormsen, G., Singh, B.R. and Jeng, A.S. 1995 Cadmium concentration in vegetable crops grown in a sandy soil as affected by Cd levels in fertilizer and soil pH. Fertilizer Research 41, 27-32.
- Hatch, D.J., Jones, L.H.P. and Burau, R.G. 1988 The effect of pH on the uptake of cadmium by four plant species grown in flowing solution culture. Plant and Soil 105, 121-126
- He, Q.B. and Singh, B.R. 1994 Crop uptake of cadmium from phosphorus fertilizers.
- II. relationship with extractable soil cadmium. Water, Air, and SoilPollution 74, 267-280.
- Hellström, L. Persson, B., Brudin, L., Petersson Grawé, K., Öborn, I. and Järup, L. 2007 Cadmium exposure pathways in a population living near a battery plant. Science of the Total Environment 373, 447-455.
- Hooda, P.S., McNulty, D., Alloway, B.J. and Aitken, M.N. 1997 Plant availability of heavy metals in soils previously amended with heavy applications of sewage sludge. Journal of Science in Food and Agriculture 73, 446-454.
- Jackson, A.P. and Alloway, B.J. 1991 The bioavailability of cadmium to lettuce and cabbage in soils previously treated with sewage sludges. Plant and Soil 132: 179-186, 1991.
- Janssen, N.A.H., Schwartz, J., Zanobetti, A., and Suh, H.H. 2002 Air conditioning and source-specific particles as modifiers of the effect of pm10 on hospital admissions for heart and lung disease. Environmental Health Perspectives 110 (1), 43-49.
- Johnson, D.E., Kienholz, E.W., Baxter, J.C., Spangler, E. and Ward, G.M. 1981heavy metal retention in tissues of cattle fed high cadmium sewage sludge. Journal of Animal Science 52, 108-114.
- Kingham, S., Briggs, D., Elliott, P., Fischer, P. and Lebret, E. 2000 Spatial variations in the concentrations of traffic-related pollutants in the indoor and outdoor air in Huddersfield, England. Atmospheric Environment 34, 905-916.
- Korentajer, L. Stern, R. and Aggasi, M . 1993 Slope effects on cadmium load of eroded sediments and runoff water. Journal of Environmental Quality 22 (4), 639-645.
- Krauss, M., Wilcke, W., Kobza, J. and Zech, W. 2002 Predicting heavy metal transfer from soil to plant: potential use of Freundlich-type functions. Journal of Plant Nutrition and Soil Science 165, 3-8.
- Lang, B. and Allin, R. 2006 Special study into the economics of cereal production 2004. Cambridge: University of Cambridge, Department of Land Economy.
- Layton, D.W. 1993 Metabolically consistent breathing rates for use in dose assessments. Health Physics 64, 23–6.
- Li, Z. and Shuman, L.M. 1996 Heavy metal movement in metal-contaminated soil profiles. Soil Science161(10), 656-666.
- Marshall, J.D., Teoh, S.K. and Nazaroff, W.W. 2005 Intake fraction of nonreactive vehicle emissions in US urban areas. Atmospheric Environment 39 (7), 1363–1371.
- McBride, M.B. 2002 Cadmium uptake by crops estimated from soil total Cd and pH. Soil Science. 167(1), 62-67.
- Milacic, R and Dolinsek, F. 1994 Direct determination of cadmium and lead in aerosols in the Zasavje region in Slovenia employing electrothermal atomic absorption spectrometry. The 2nd International Conference on Air Pollution. Part 1 (of 2), Barcelona, Spain, 09/27-29/94; pp. 377-384.
- Milner, J.T., Dimitroulopoulou, C. and ApSimon, H.M. 2004 Indoor concentrations in buildings from sources outdoors. Contract Report for ADMLC (ADMLC/2004/2).
- Mueller, M. and Anke, M 1994 Distribution of cadmium in the food chain (soil-plant-human) of a cadmium exposed area and the health risks of the general population. Science of the Total Environment. 156 (2), 151-158.
- [www.statistics.gov.uk National Statistics 2002]. Key findings of the UK 2000 time use survey.
- Olsson, I-M., Eriksson, J., Öborn, I., Skerfving, S. and Oskarsson, A. 2005 Cadmium in food production systems: a health risk for sensitive population groups. Ambio34 (4–5), 344-351.
- Ryan, P.B., Huet, N. and MacIntosh, D.L. 2000 longitudinal investigation of exposure to arsenic, cadmium, and lead in drinking water. Environmental Health Perspectives. 108 (8), 731-735.
- Sharma, R.P., Street, J.C., Verma, M.P. and Shupe, J.L. 1979 Cadmium uptake from feed and its distribution to food products of livestock. Environmental Health Perspectives. 28, 59-66.
- Smilde, K.W., van Luit, B. and van Driel, W. 1992 The extraction by soil and absorption by plants of applied zinc and cadmium. Plant and Soil 143: 233-238.
- Spadaro, J.V. and Rabl, A. 2004 Pathway analysis for population-total health impacts of toxic metal emissions. Risk Analysis 24, 1121-1141.
- van Bruwaene, R., Kirchmann, R. and Impens, R. 1984 Cadmium contamination in agriculture and zootechnology Experientia 40, 4-52.
- van Driel, W. and Smilde, K.W. 1990 Micronutrients and heavy metals in Dutch agriculture. Fertilizer Research 25, 115-126.
- van Lune, P. and Zwart, K.B.1997 Cadmium uptake by crops from the subsoil. Plant and Soil 189, 231–237.
- Vidovic, M., Sadibasic, A., Cupic, S. and Lausevic, M. 2005 Cd and Zn in atmospheric deposit, soil, wheat, and milk. Environmental Research 97, 26-31.
- Wallace, L. 1996 Indoor particles: a review. Journal of the Air and waste Management Association 46, 98-126.
- Williams, C.R. and R. M. Harrison 1984 Cadmium in the atmosphere. Experientia 40, 30-36.