Internal dose estimation in IEHIAS
- The text on this page is taken from an equivalent page of the IEHIAS-project.
The risk to human health posed by a chemical agent is not fixed at the moment, or by the amount, of exposure. It depends, also, on what happens thereafter as it enters into, and is absorbed by, the body: the uptake-elimination processes, the associated changes in the chemistry of the agent, and eventually the contact between the biologically-active form of the chemical and the critical target receptor in the body that elicits a toxic response. These processes vary greatly between individuals, and between different circumstances, so are a major source of variability in the ultimate health effects seen across a population. To understand these variations, therefore, often requires information on the internal (i.e. biological) fate of contaminants.
Two primary approaches are available to elicit this information:
- Human biomonitoring provides measured data on dose at different points in the intake-uptake chain, and can thus be used to assess variations, or track changes, in intake and uptake across a population;
- Pharmacokinetic and toxicokinetic models provide a means of analysing and simulating the processes involved, and thus of predicting changes in dose under different scenarios.
Both are integral to understanding the mechanisms by which toxicants affect human health; both, also, play an important role in health impact assessments, by taking account of the biological factors that intervene between external exposure to an agent and its health effects.
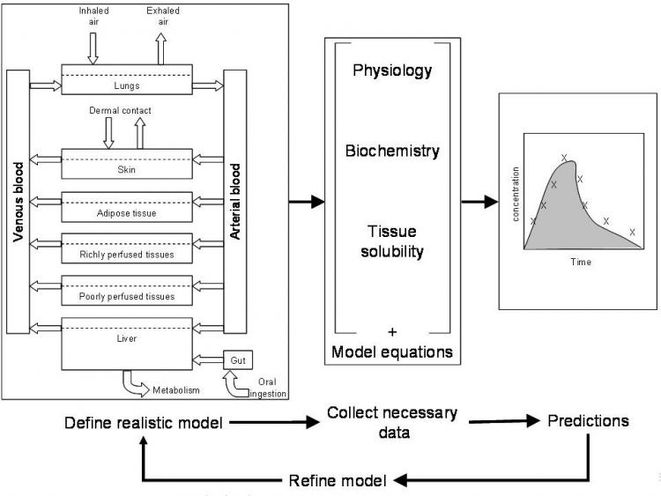
PBPK modelling
Human biomonitoring provides an important source of information on dose, and new advances in biomonitoring have greatly extended the range of agents and associated health effects that can be assessed. In most cases, however, biomonitoring data provide aggregate measures of dose across all sources and exposure pathways. As such biomonitoring does not, of itself, enable the different exposure pathways to be identified and quantified, nor can it show how the dose will change if the levels of exposure via these various pathways change, under different scenarios. For these purposes, other forms of information, including modelling, need to be used. One way of doing this is through the use of physiologically-based pharmacokinetic (PBPK) or toxicokinetic (PBTK) models.
Principles of PBPK modeling
In recent years, PBPK modeling has been successfully applied to calculate tissue doses of chemicals and their metabolites over a wide range of exposure conditions. Because these models are based on the human physiology and anatomy and summarise the behaviour of chemicals in the body, they are often considered to be more realistic than empirical models. In PBPK models, the body is subdivided into a series of compartments that represent specific organs, or lumped tissue and organ groups, with appropriate volumes, blood flow rates, and pathways of metabolism. Routes of exposure and intake are included, in their proper relationship to the overall physiological structure, and changes in exposure under the scenarios being considered are accounted for in the time sequence of the dose input terms. The transfer of chemicals between the different compartments is described by differential equations, with perfusion being the main limiting parameter for distribution of the chemical within a given compartment (Jonsson 2001).
In principle, it might be expected that a model of this sort could be readily transferred between different individuals and situations. This would be possible if any two identical individuals (human or animal) responded identically when in contact with the same agent in their environment. In this case, estimation of uptake and dose, would be straightforward: a particular exposure would lead to a related internal dose, which could be determined by modelling or human biomonitoring, and this in turn would cause an almost predefined health effect. In the real world, however, this is not the case. Because of differences in exposure profiles among different individuals and populations, and inter-individual variability in pharmacokinetic characteristics, different organisms respond differently to their environment and in the way they process chemicals within the body. Modelling of dose, and interpretation of the importance of different exposure routes and metabolites to the dose, thus need to take account both of the environmental exposures that lead to intake of the agent, and the physiological attributes of the exposed individuals, which determine the fate of the agent once it enters the body.
Data on the contact environment are usually obtained in the form or measured or modelled concentrations of the chemical in the relevant environmental compartments (air, water, soil, food), or micro-environments, In addition, information on specific behaviours (e.g. smoking) may be used as proxies for micro-environmental (especially indoor) concentrations or amounts received via water or food. Information on the physiological and physiochemical characteristics that control toxicokinetic processes are more difficult to obtain, and this contributes to uncertainties in model parameterisation. PBPK models therefore need to be carefully calibrated to ensure that the results are consistent with toxicokinetic measurements, and validated against human biomonitoring data in order to disentangle the (biologically real) variability in the inter-individual toxicokinetic behaviour from uncertainty in the model parameters and input data. Recently, the more sophisticated models have applied Bayesian methods to take account of population variability in pharmacokinetic parameters, mainly using a Bayesian approach.
Some generic PBPK models
Lack of suitable toxicokinetic data has meant that the application of full, validated PBPK models for the purpose of risk assessment has been limited. Generic toxicokinetic models have, however, been developed which allow for a rapid screening-level approach to PBPK modeling. Three such models are summarised below:
- IndusChemFate is a generic PBTK model, developed as part of a CEFIC LRI-funded project by IndusTox Consult. Its purpose is to derive human biomonitoring equivalent guidance values (BEGV) for multiple (data-poor) chemicals. It is a first tier or screening tool that requires a minimum of input data. It contains algorithms representing Quantitative Structure-Property Relationships (QSPRs) for blood:air and tissue:blood partitioning. These enable the model to provide useful information even when experimental partition characteristics of a compound are lacking. The model is programmed in Visual Basic and runs in MS Excel. Data input is via two specifically-designed Excel worksheets, and output comprises a numerical listing and graphs in the same Excel-file. The model is provided as freeware with an open source code.
- MEGen (Model Equation Generator) is a 'proof of concept', intuitive user interface for the rapid generation and analysis of PBPK-models, developed by UK Health and Safety Laboratory. MEGen enables a user to describe physiology, biology and toxicology in order to derive a set of mathematical equations that emulate the information supplied by the user and constitute a PBPK model. During this process, the software interrogates a built-in database, supplying pertinent data for use within the model. The resulting mathematics can be translated and imported into a number of commercial modeling packages, for the purpose of visualisation and implementation. MEGen provides a schematic diagram of the built model along with a corresponding table listing the value, units, source, origin and reference for each parameter specified. The diagrams and tables can be exported directly into documents prepared in standard word processors. MEGen is freely available under the General Public License regulation.
- PKQuest is a slightly older program, which includes a “ Standard human” and “Standard rat” data set. It has a relatively simple user interface and graphical output, and has recently been revised as a Java application (PKQuest_Java) (Levitt 2002). PKQuest_Java is designed for the non-specialist who would like to attempt some PBPK modeling without acquiring detailed training or expensive software. It is freely available along with a range of detailed examples.
Additional resources
A report on "Approaches for the application of physiologically based pharmacokinetic (PBPK) models and supportinig data in risk assessment", which is promoted as a definitive desk reference and learning tool for risk assessors and risk managers who are interested in evaluating and usinig PBPK models in their work, is avialable from the following USEPA website: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=157668
A second report focuses more on the impact of human age and inter-individual differences in physiology and biochemistry pertinent to risk. this document includes tutorial materials, including case studies on trichloroethylene and chloroform, and can be found at the following location: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=151384
Finally, a database containing human physiological data for adults aged 60 and over is available at the following location: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=201924
References
- Blaauboer, B.J. 2003. The integration of data on physico-chemical properties, in vitro-derived toxicity data and physiologically based kinetic and dynamic as modeling tool in hazard and risk assessment. a commentary. Toxicology Letters 138, 161-171.
- Beaudouin, R., Micallef, S. and Brochot, C. 2010 A stochastic whole-body physiologically based pharmacokinetic model to assess the impact of inter-individual variability on tissue dosimetry over the human lifespan. Regulatory Toxicology and Pharmacology 57, 103-116.
- Bois, F.Y. 2001 Applications of population approaches in toxicology. Toxicology Letters 120, 385-394.
- Dahl, S.G., Aarons, L., Gundert-Remy, U., Karlsson, M.O., Schneider, Y.-J., Steimer, J.-L. and Troconiz, I.F. 2009 Incorporating physiological and biochemical mechanisms into pharmacokinetic-pharmacodynamic models: a conceptual framework. Basic and Clinical Pharmacology and Toxicology 106, 2-12.
- Jonsson, F. 200. Physiologically based pharmacokinetic modeling in risk assessment: development of Bayesian population methods. Uppsala: Uppsala University, Division of Pharmacokinetics and Drug Therapy, 46pp.
- Levitt, D.G. 2002 PKQuest: a general physiologically based pharmacokinetic model. Introduction and application to propranolol. BMC Clinical Pharmacology 2, 21pp.
- Lipscomb, J.C., Meek, M.E., Krishnan, K., Kedderis, G.L., Clewell, H. and Haber, L. 2004 Incorporation of pharmacokinetic and pharmacodynamic data into risk assessments. Toxicology Mechanisms and Methods 14, 145-158.
- Loizou, G., Spendiff, M., Barton, H.A., Bessems, J., Bois, F.Y., Bouvier d’Yvoire, M., Buist, H., Clewell, H.J., Meek, B., Gundert-Remy, U., Goerlitz, G. and Schmitt, W. 200. Development of good modeling practice for physiologically based pharmacokinetic models for use in risk assessment: the first steps. Regulatory Toxicology and Pharmacology 50, 400-411.]
- Lyons, M.A., Yang Computational Toxicology of Chloroform: Reverse Dosimetry Using Bayesian Inference, Markov Chain Monte Carlo Simulation, and Human Biomonitoring Data
- Mayeno, A.N. and Reisfeld, B. 2008 Computational toxicology of chloroform: reverse dosimetry using Bayesian inference, Markov Chain Monte Carlo simulation, and human biomonitoring data. Environmental Health Perspectives 116, 1040-1046.
- Sohn, M.D., McKone, T.E. and Blancato, J.N. 2004 Reconstructing population exposure from dose biomarkers: inhalation of trichloroethylene (TCE) as a case study. Journal of Exposure Analysis and Environmental Epidemiology 14, 204-213.
See also
- Exposure, intake and dose models
- PBPK model in assessing population exposure to cadmium
- Piloting: Finding and evaluating data#Human biomonitoring data
- Human biomonitoring: methods