Non-parametric Bayesian estimation
| Moderator:Jouni (see all) |
|
|
| Upload data
|
We present here the nonparametric Bayesian approach to uncertainty modeling in dose response. The Bayesian approach is unique in that Bayes’s theorem provides the mechanism to combine data with the prior information to produce updated results in the form of posterior distributions, a combination of the prior distribution (derived using prior information) and the likelihood function derived from the data.
In our analysis, we assume that the number of responses, Si, when 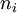 subjects are exposed to dose level xi are independently distributed as Binomial random variables, Bin(ni,pi), where ni is the number of experimental subjects at dose level xi, and pi=P(xi) is the unknown probability of response at dose level xi. Thus, the likelihood at S=s is
subjects are exposed to dose level xi are independently distributed as Binomial random variables, Bin(ni,pi), where ni is the number of experimental subjects at dose level xi, and pi=P(xi) is the unknown probability of response at dose level xi. Thus, the likelihood at S=s is
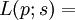 (1.1)
(1.1)
where , and M is the number of experiments. ),,
The prior belongs to the nonparametric class of right continuous, non-decreasing functions taking values in [. We introduce the Dirichlet Process (DP) as a prior distribution on the collection of all probability measures. We define it as follows: a random probability distribution P is generated by the DP if for any partition of the sample space, the vector of random probabilities follows a Dirichlet distribution:
ADD LATEX CODE
Note that in order to specify DP prior, a precision parameter 0>α and base distribution are required. Here, defines the prior expectation:
the parameter α controls the strength of belief, or confidence in the prior. Thus, a large (small) value of α means that new observations will have a small (large) affect in updating the prior . Indeed, 0Pα is sometimes interpreted as the ‘equivalent prior observations’. In our study, we assume that P has an Ordered Dirichlet distribution, with density at ()1,,Mpp…as stated below:
ADD LATEX CODE
(1.2)
where and
ADD LATEX CODE
Unfortunately the joint posterior distribution, obtained from (1.1) and (1.2), is too complicated to allow an analytical solution, especially for obtaining the marginals. Therefore, we introduce one of the most commonly used Markov Chain Monte Carlo methods - the Gibbs sampler, which can be used to generate the marginal posterior densities. We follow investigation of Gelfand and Kuo (1991). The Gibbs Sampler requires sampling from the following conditional distributions successively resample the following conditional distributions:
ADD LATEX CODE
(1.4) where 11(,,,,)jMλλλλ+=…… with 1jjjppλ−=−, The variable denotes, amongst the individuals receiving dosage level , the unobserved number of subjects who would have responded to dosage level but not to dosage level. Notice that is a concatenation of two multinomials ijZinixjx1−jxiZ)}2(),1({iiiZZZ=such that:
ADD LATEX CODE
(1.5) The posterior mean of is estimated using the mean of leading to ipDg
ADD LATEX CODE
(1.6) for . Mi,,1…= As we have mentioned, our sampling procedure is conducted with v parallel replications each taken to r iterations. Note that the choice of v determines how close our density estimates is to the exact density at the r-th iteration, whereas the choice of r determines how close the density estimate at r-th iteration is to the actual marginal posterior density. Therefore, values for r and v to obtain smooth convergent estimates are strictly dependent on the application and the prior information about the studied problem. If we are interested in other dose levels than those in our bioassay experiments, we will have to run the Gibbs sampler for each new dose level.
References
Burzala L, Mazzuchi T. (2007). Uncertainty Modeling in Dose Response Using Non-Parametric Bayes: Bench Test Results. Lidia Burzala Delft University of Technology, The Netherlands Thomas A. Mazzuchi George Washington University, Washington DC link
1. AMMANN,L.P. (1984). Bayesian nonparametric inference for quantal response data.Ann. Statist.12, 636-45
2. ANTONIAK,C.E.(1974). Mixtures of Dirichlet process with applications to Bayesian nonparametric problems. Ann.Statist. 2,1152-74.
3. AYER,M.,BRUNK,H.D.,EWING,G.M.,REID,W.T.,SiILVERMAN,E.(1955). An empirical distribution function for sampling with incomplete information. Ann.Math.Statist.,26,641-7
4. BHATTACHARYA,P.K.(1981). Posterior distribution of a Dirichlet process from quantal response data. Ann.Statist.9,803-11
5. DISCH,D.(1981). Bayesian nonparametric inference for effective doses in quantal response experiment. Biometrics 37,713-22
6. FERGUSON.T.S.(1973). A Bayesian analysis of some nonparametric problems. Ann.Statist.1,209-230
7. FINNEY, D.J. (1978). Statistical Methods in Biological Assay
8. GEFLAND, A.E., KUO, L. (1991). Nonparametric Bayesian bioassay including ordered polytomus response. Biometrica,78,3,pp.657-66
9. GEFLAND, A.E., SMITH, A.F.M.(1990). Sampling based approach to calculating marginals densities. J.Amer.Statist. Assoc.,85,398-409
10. GOVINDARAJULU,Z. (1988) Statistical techniques in bioassay. S. Karger Publishers (USA) (2nd edition)
11. MUKHOPADHYAY,S.(2000). Bayesian nonparametric inference on the dose level with specified response rate. Biometrics 56,220-226
12. MÜLLER P. QUINTANA F.A. (2004). Nonparametric Bayesian data analysis. Satatist.Sci.19, no.1, 95-110
13. RAMGOPAL,P., LAUD,P.W., SMITH,A.F.(1993). Nonparametric Bayesian bioassay with prior constrains on the shape of the potency curve. Biometrika,80,3,pp.489-98
14. RAMSEY,F.L. (1972). A Bayesian approach to bio-assay. Biometrics 28, 841-58
15. SHAKED,M. SINGPURWALLA,N.D.(1990). A Bayesian approach for quantile and response probability estimation with applications to reliability. Ann.Inst.Statist.Math.42,1-19
