Cinnamomun verum
| Moderator:Olli (see all) |
| This page is a stub. You may improve it into a full page. |
| Upload data
|
Scope
This document presents a draft for assessing benefits and risks of cinnamon, (Cinnamomum verum). The starting point is to agree on what are the health endpoints to include. Most of the non-clinical data available are related to cinnamaldehyde. When using the essential oil or extracts of cinnamon the results are mostly negative. In summary the data on toxicity is insufficient. Indications in folk medicine have not been sufficiently documented (Hänsel et al., 1992). Despite of the long tradition of use, the bark and the essential oil of Cinnamomum verum do not fulfill the requirements of a well-established medicinal use with recognised efficacy. Further investigation as to the beneficial role of the natural matrix is required. Proposed human health endpoints are gathered from EMA 2010 and here is a summary of the findings:
Key adverse health effects of cinnamaldehyde:
- Liver toxicity (sensitive population subgroup)
- dermatitis (cinnamaldehyde) (non-clinical)
Beneficial health effects of cinnamaldehyde (results only from clinical studies presented):
- fasting serum glucose reduction (18-29%) (Cinnamomum cassia)
- triglyceride reduction (23-30%) (Cinnamomum cassia)
- LDL cholesterol reduction (7-27%) (Cinnamomum cassia)
- total cholesterol reduction (12-26%) (Cinnamomum cassia)
- glucose homeostasis (Solomon and Blannin, 2007))
- reduces risk factors associated with diabetes and cardiovascular disease (Roussel et al., 2009) (Cinnamon cassia)
Purpose
The purpose of this document is to provide insight on how benefit-risk assessment are performed and what are the data needs.
Definition
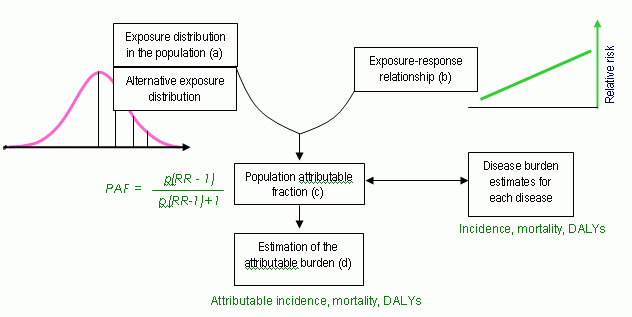
Comprehensive risk assessment requires detailed data. The procedure from the Environmental Burden of Disease in Europe (EBoDE) project is described in figure 1 (Full description can be found in EBoDE 2010). The data needed for a complete assessment is 1) exposure distribution, 2) exposure-response relationship (relative risk RR), 3) severity weight, and 4) duration of disease.
Figure 1. Data needs in DALY assessment
Result
The difficulty is that all data above is hardly ever available for plant food supplements. Therefore we should think of a lighter version describing a) severity of health outcome (severity weight) and b) probability of the outcome. These two together form a general definition of risk. Illustration of this method is presented in table 1. Obviously, the estimation is not fully quantitative by nature, and actual estimates for require specific data on population and disease. Translating these values into DALY requires yet additional data on disease and exposure-response function. Benefits are presented in a similar way in table 2.
Table 1. Characterisation of risk using DALY when complete data is not available. Note, the figures here are arbitrary and serve only demonstrative purposes.
| Plant | Compound | Health endpoint | Severity weight | Exposed population estimation |
|---|---|---|---|---|
| Cinnamon verum | cinnamaldehyde | Liver toxicity | 0.12 | < 0.05% |
| Cinnamon verum | Cinnamaladehyde | Dermatitis | 0.02 | < 0.1% |
Health benefits of plant food supplements are typically not disease driven health endpoints but rather value driven ones. This causes traditional risk assessment methods to become inefficient. This is discussed in detail in valuation of health impacts page. Table 2 presents two health benefits calculation as examples. These are examples of traditional disease avoiding driven health endpoints. These can also be translated into DALY estimation with additional data on disease and exposure-response function. Closer description of calculation is described in (EBoDE 2010)
Table 2. Characterisation of benefit using avoided severity. Values are demonstrative, more rigorous assessment needed for closer evaluation.
| Plant | Compound | Health endpoint | Severity weight avoided | Exposed population of a disease |
|---|---|---|---|---|
| Cinnamon cassia | Cinnamaladehyde | Reduced risk factors associated with diabetes | 0.256 Unweighted average using diabetes symptom's severity weights | Prevalence of diabetes in a country (population subgroup potentially benefiting) |
| Cinnamon cassia | Cinnamaladehyde | Reduced risk factors associated with cardiovascular disease | 0.184 Unweighted average using cardiovascular symptom's severity weights | Prevalence of cardiovascular diseases in a country (population subgroup potentially benefiting) |
The alternative way of evaluating the health effects, proposed in the PlantLIBRA milestone 5.5 [1], is to report incidence. For benefits it can be calculated if country-specific disease incidence and exposure & exposure-response function is known. The calculation is described in detail in EBoDE calculation method description (EBoDE 2010).
Conclusions
This document presents a descriptive way of expressing risk of cinnamon use. With xposure data and exposure-response relationships the results can be translated into DALYs.
See also
Common currency in health assessments: http://en.opasnet.org/w/Common_currency_in_health_assessments
References
EBoDE. 2010. Calculation method on password protected project site: http://heande.opasnet.org/wiki/File:EBoDE_General_EBD_Methodology.doc Calculation method direct link [2]
EMA. 2010. European Medicines Agency. Assessment report on Cinnamomum verum J. S. Presl (Cinnamomum zeylanicum Nees), cortex and corticis aetheroleum. Committee on Herbal Medicinal Products (HMPC). 15 July 2010 EMA/HMPC/246773/2009.