Plantlibra deliverable DWP5-6
| Number | DWP5-6 (Task 5.9) |
| Due | M46 (Mar 2014) |
| Description | Database interface for risk-benefit assessment |
| Nature | P (Prototype) |
| Dissemination level | PP (Restricted to project partners and the Commission) |
| Responsible person | THL |
Background and objective of the work
The use of plants and plant derivatives to maintain health has been popular throughout Europe for many centuries. The consumption of teas, digestive drinks, juices, elixirs and extracts prepared from botanicals and used for health maintenance purposes has become part of European cultural heritage. Plant food supplements are a modern-day extension of this process.
PlantLIBRA project (PLANT food supplements: Levels of Intake, Benefit and Risk Assessment) aims to foster the safe use of food supplements containing plants or botanical preparations, by increasing science-based decision-making by regulators and food chain operators. To make informed decisions, competent authorities and food businesses need more quality-assured and accessible information and better tools (e.g. metadata banks). [1]
PlantLIBRA is structured to develop, validate and disseminate data and methodologies for risk and benefit assessment and implement sustainable international cooperation. International cooperation, on-spot and in-language capacity building are necessary to ensure the quality of the plants imported in the EU.
The Description of Work for PlantLIBRA project describes the objective of this deliverable in the following way:
Task 5.9: Integration of databases and guidance
Taking into account the database of WP6, THL will develop an Internet-based interface (DWP5-6) where users can access, combine, by plant and compound, and discuss information for risk-benefit assessment. In this context, THL will make selected case-studies available to the public through the Mediawiki platform, when possible. THL will develop guidance for conducting risk-benefit assessments of plants, extracts, preparations and individual products which will be made available online as Deliverable 5-6 (DWP5-6).
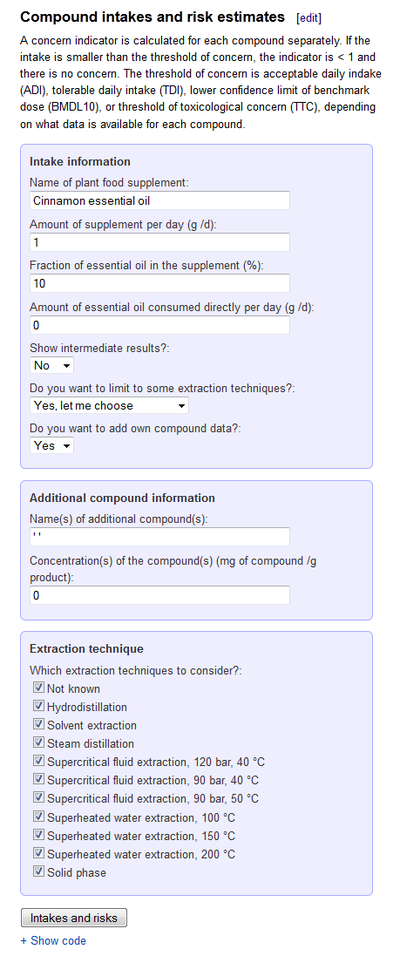
Compound intake estimator
Compound intake estimator calculates intakes of compounds based on food or food supplement intake, compound concentration in food, and guidance values for the compound. In the text, we talk about plant-based food supplements (PFSs), but the tool is generic and is usable for other products as well as long as the required concentration data is made available.
Question
How to estimate intakes of chemical compounds in plant-based food supplements and compare them to guidance values in an open collaborative web system?
Answer
The compound intake estimator calculates intakes and levels of health concern of plant-based food supplements based on product-specific concentration data and usage information provided by the user. The tool works online simply with a web browser. For user instructions and some data used, see below. The tools is openly available online at http://en.opasnet.org/w/Compound_intake_estimator.
Compound intakes and risk estimates
A concern indicator is calculated for each compound separately. If the intake is smaller than the threshold of concern, the indicator is < 1 and there is no concern. The threshold of concern is acceptable daily intake (ADI), tolerable daily intake (TDI), lower confidence limit of benchmark dose (BMDL10), or threshold of toxicological concern (TTC), depending on what data is available for each compound.
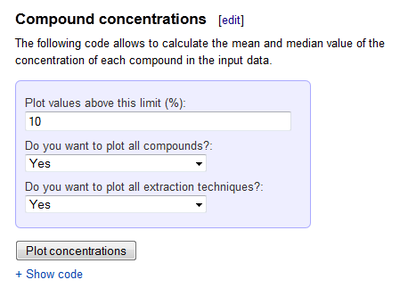
Compound concentrations
The following code allows to calculate the mean and median value of the concentration of each compound in the input data.
For seeing details of what the code actually does, click Show code. The code will appear, with documentation of each part on rows starting with ###. In brief, the code reads a piece of concentration and other data from Opasnet database, selects the rows defined by the user through the interface above, and shows the data on a summary table and a few graphs.
Rationale
For successful benefit-risk assessment of plant-based food supplements (PFSs), there is a need to estimate intakes of relevant compounds in a product. The health impacts of this exposure are then estimated based on available exposure-response functions. Optimally, all health impacts can be aggregated into e.g. disability-adjusted life years (DALYs) using disability weights or similar importance weights. However, the tool presented here does not go so far. Rather, it compares the intakes of each relevant compound to a guidance value, which is thought to separate safe intakes from non-safe intakes. Non-safe does not mean that the compound is causing health problems; it merely tells about compromised safety margins that are inherently embedded in the guidance values. Small exceedances are most likely harmless, but if the intake is clearly beyond its guidance value, careful consideration of potential risk is warranted.
This web tool, Compound intake estimator, was developed in Plantlibra project to promote the use of quantitative estimates of compound intakes and health risks. The tool consists of open data about guidance values (in the Opasnet database), an open source model (running on R software) to compute intakes and risk estimates, product-specific concentration data that is only partially available, and a user interface where the user can choose modelling options and add case-specific compound concentration data as input to the model. Detailed user guide, and the data and the model are available at http://en.opasnet.org/w/Compound_intake_estimator.
Users who are skilful with R can download the whole model to their own computers and use and develop it further as they please. This requires an R package called OpasnetUtils, which is available from the CRAN repository http://www.cran.r-project.org. It is also possible to develop a new page in Opasnet for a new product and its concentration data. Those who are interested in such work should contact the tool developer Jouni Tuomisto at THL, Finland.
Guidance values
There are several possible approaches for deriving guidance values. In this approach, we use acceptable daily intake (ADI), tolerable daily intake (TDI), lower confidence limit for benchmark dose 10 (BMDL10), and threshold for toxicological concern (TTC). If there are several guidance values available for a single compound, they are used in the preference order mentioned here.
- Acceptable daily intake (ADI)
- An estimate of the amount of a chemical or toxin that a person can ingest daily over a human’s lifetime without appreciable health risk, divided by an average person’s lifespan. The concept of the ADI was developed by the WHO and FAO for chemicals such as additives to foods, pesticide residues and veterinary drugs in foods; ADIs are derived from lab toxicity data and from human experiences of such chemicals when available, and incorporate a safety factor; the ADI is an estimate of the amount of a substance in food that can be ingested over a lifetime by humans without significant risk to health.[2]
- Tolerable daily intake (TDI)
- Refers to the daily amount of a chemical that has been assessed safe for human being on long-term basis (usually whole lifetime). Originally acceptable daily intake (ADI) was introduced in 1961 to define the daily intake of a food additive which, during the entire lifetime, appears to be without appreciable risk. For contaminants and other foreign chemicals not used intentionally, the term TDI is often preferred.[3]
- Lower confidence limit for benchmark dose 10 (BMDL10)
- For the dose-response function of a health impact, it is possible to estimate the dose that causes on average 10 % response compared with the highest achievable response. This is called the benchmark dose (BMD10). BMDL10 is the lower confidence limit of BMD10, typically the 2.5th percentile of the probability distribution of the BMD10 estimate.[4]
- Threshold for toxicological concern (TTC)
- According to the TTC concept, a "safe" level of exposure can be identified for many chemicals based on their chemical structure and the known toxicity of chemicals that share similar structural characteristics. The TTC approach is exclusively designed as a substitute for substance-specific information in situations where there is limited or no information on the toxicity of the compound and information on exposure indicates that human exposure is very low.[5]
If a compound in a PFS does not have any information on these guidance values, it is ignored in this analysis. The same happens to compounds that exist in the product but do not have any concentration information.
Intake and guidance value calculations
The total intake of essential oil is calculated like this:
intakeoil = amountsuppl * fractionoil + amountoil
where
- intake is the total intake of essential oil (or another product part with the compounds of interest)
- amountsuppl is the intake of food supplement
- fractionoil is the fraction of essential oil in the food supplement
- amountoil is the intake of the essential oil consumed directly, i.e. in addition to what is in the food supplement.
The intakes of individual compounds are calculated like this:
intakecomp (mg /kg /d) = intakeoil (g /d) * conccomp (mg /g) / 60 (kg)
where
- intakecomp are the intakes of each compound
- intakeoil is the total intake of essential oil (or another product part with the compounds of interest)
- conccomp are the concentrations of each compound in the essential oil.
- 60 kg is the assumed body weight of the person
The concern indicator is calculated like this:
concern indicatorcomp,i = intakecomp (mg /kg /d) / guidance valuecomp,i (mg /kg /d) * safety factori
where
- concern indicatorcomp,i are the preferred indicators for each compound,
- guidance valuecomp are the guidance values for each compound,
- safety factori are the safety factors used for each indicator (10000 for BMDL10, 1 for others, which already include a safety factor)
The concern indicator is calculated for each compound separately. If the intake is smaller than the guidance value, the indicator is < 1 and there is no concern. Only one guidance value indicator i is used for each compound, the preference order (when there are several guidance values for a compound) is explained in the previous section.
For BMDL10, the typical approach is to divide BMDL10 by the intake and interpret values > 10000 as safe. However, it would be confusing for the user to treat one guidance value differently than others. Therefore, this tool applies the same equation to all guidance values and uses 10000 as the default safety factor for BMDL10. For all other guidance values, the default safety factor is 1, because they already have embedded safety factors for interspecies and intraspecies variation.
User interface
You can fill in the following information:
- Name of plant food supplement (PFS) product. If this is given, it is shown on the graphs and outputs as title.
- Fraction of essential oil in the supplement. How much (as a percentage) does the total product contain the essential oil?
- Amount of supplement per day. How much is the supplement (the total product) consumed per day (given as grams of product per day)?
- The compound concentration data is taken from a table in the Opasnet database. However, if you know that the product contains something that is not listed in the table, you may add the information here.
- Name of additional compound. The name is used to combine the concentration data with toxicological data. So, the name should be found in one of these two tables on this page: Cramer classes of compounds, or Guidance values. If the name is not found, the new piece of information is ignored.
- Concentration of the additional compound in the supplement. This is given as mg of compound per g of PFS product.
- Technical note: You should enter the name of a single compound between quotation marks, e.g. 'Estragol'. If you add several compounds, you should use vector formatting for R, e.g. c('Estragole', 'Fenchone'). The concentration value can be without quotation marks unless it is a distribution or a vector. Acceptable entries for concentrations are e.g.: 2.1; '21.4-24.5'; and c('2.1', '21.4-24.5').
- Show intermediate results? If Yes is selected, you will get two tables of a) concentration and b) intake estimates with all compounds in the product. Otherwise, only a default output is shown (see below).
- Which extraction techniques to consider? Different extraction methods may result in different concentration estimates even from the same product. Some of the toxicological information is given having a particular extraction method in mind, and it is not always possible to use toxicological information with any concentration information. Therefore, if you know that some extraction methods should not be used to estimate toxicology, you can unselect them from the list. The default is to use all data, but you can select Yes, let me choose to the extraction techniques, and then select from the list that appears.
- The default outcome shows a table and a graph where the level of concern is shown for each compound. However, because there are dozens of compounds in any product, the graph shows only those that have the level more than one, i.e. those compounds that need further scrutiny.
Concentrations
The concentration code allows to calculate the mean and median value of the concentration of each compound that appear in table. In addition, it is possible to plot the concentration (expressed as percentage of essential oil) of each compound reported in the data table. Each single value is reported in the chart.
Data
This is an example data to show what kind of information is used by the tool. It is also used to calculate the example results. Some of the actual data for PFSs is proprietary and is currently only available to the partners of the Plantlibra project.
| Obs | Extraction.technique | Compound | Result | Reference |
|---|---|---|---|---|
| 1 | Superheated water extraction, 100 °C | 2-Carene | 0.08; 0.1; 0.12 | Jayawardena & Smith, 2010 |
| 2 | Superheated water extraction, 100 °C | Z-Cinnamaldehyde | 1.63; 2.1; 2.57 | Jayawardena & Smith, 2010 |
| 3 | Superheated water extraction, 100 °C | Cinnamaldehyde | 81.14; 83.7; 86.26 | Jayawardena & Smith, 2010 |
| 4 | Superheated water extraction, 100 °C | Eugenol | 0.45; 0.8; 1.15 | Jayawardena & Smith, 2010 |
| 5 | Hydrodistillation | 1,8-Cineole | 0.2 | Chericoni et al, 2005 |
| 6 | Superheated water extraction, 100 °C | Cinnamyl acetate | 5.47; 7.2; 8.93 | Jayawardena & Smith, 2010 |
Calculations
- This is the code for the actual compound intake estimator model. You need to run the code below only if you update the guidance value tables on this page.
Using different data on different layers: [7].
Extraction techniques
| Obs | Extraction.technique | Notes |
|---|---|---|
| 1 | Not known | |
| 2 | Hydrodistillation | |
| 3 | Solvent extraction | |
| 4 | Steam distillation | |
| 5 | Supercritical fluid extraction, 120 bar, 40 °C | |
| 6 | Supercritical fluid extraction, 90 bar, 40 °C | |
| 7 | Supercritical fluid extraction, 90 bar, 50 °C | |
| 8 | Superheated water extraction, 100 °C | |
| 9 | Superheated water extraction, 150 °C | |
| 10 | Superheated water extraction, 200 °C | |
| 11 | Solid phase | |
| 12 | 70% ethanol (1:5) | |
| 13 | Acetone and water mixture (35 to 67:1) | |
| 14 | Commercial product | |
| 15 | Methanol (40%) and water | |
| 16 | Not reported | |
| 17 | Ultrasound assisted extraction | |
| 18 | 70% methanol, Ultrasound assisted extraction | |
| 19 | ddH2O and Na2HPO4,partitioning with ethyl acetate, organic phases dried and redissolved in methanol | |
| 20 | Maceration | |
| 21 | Soxhlet extraction |
Data about guidance values
Generic data about guidance values
| Obs | Guidance | Safety factor |
|---|---|---|
| 1 | ADI | 1 |
| 2 | BMDL10 | 10000 |
| 3 | TDI | 1 |
| 4 | TTC | 1 |
| Obs | Cramer class | Threshold of toxicological concern | Description |
|---|---|---|---|
| 1 | 1 | 30 | |
| 2 | 2 | 1.5 | |
| 3 | 3 | 1.5 | |
| 4 | 4 | 0.0025 | This value is the TTC for compounds having a structural alert for genotoxicity |
Values 30, 1.5 and 0.025 µg /kg /d come from EFSA (2012)[6]
Compound-specific data about guidance values
| Obs | Compound | Guidance | Result | Notes |
|---|---|---|---|---|
| 1 | 1,8-Cineole | ADI | 2.8 | |
| 2 | 4-Terpineol | ADI | 1.2 | |
| 3 | Benzylbenzoate | ADI | 5 | |
| 4 | Cinnamaldehyde | ADI | 0.7 | |
| 5 | Coumarin | TDI | 0.1 | |
| 6 | Estragole | BMDL10 | 3.3-6.5 | |
| 7 | Eugenol | ADI | 2.5 | |
| 8 | Fenchone | ADI | 10.64 | |
| 9 | Limonene | ADI | 1 | |
| 10 | Linalool | ADI | 0.5 | |
| 11 | Myrcene | ADI | 2.5 | |
| 12 | Trans-anethole | ADI | 2 | |
| 13 | Safrole | BMDL10 | 1.9-5.1 | |
| 14 | α-Terpineol | ADI | 1.2 |
| Cramer classes of compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
See also
- Plantlibra
- Plantlibra deliverable DWP5-6
- heande:Composition of cinnamon dried bark essential oil
- heande:Composition of plant-based food supplements
- heande:Composition of bitter fennel essential oil
- Compound intake estimator
 Compound intake estimator Presentation about the tool
Compound intake estimator Presentation about the tool
References
- ↑ PlantLIBRA website
- ↑ Segen's Medical Dictionary. 2012 Farlex, Inc. [1] accessed 15th April, 2014.
- ↑ Wikipedia: Tolerable daily intake accessed 15th April 2014
- ↑ U.S.EPA: Benchmark Dose (BMD) Methodology [2] accessed 15th April, 2014.
- ↑ Opinion on the Use of the Threshold of Toxicological Concern (TTC) Approach for Human Safety Assessment of Chemical Substances with focus on Cosmetics and Consumer Products. European Union (2012) SCCP/1171/08. [3] accessed 15th April 2014.
- ↑ EFSA (2012). Scientific Opinion on Exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 2012;10(7):2750
- ↑ van den Berg SJPL, Restani P, Boersma MG, Delmulle L, Rietjens IMCM (2011) Levels of Genotoxic and Carcinogenic Ingredients in Plant Food Supplements and Associated Risk Assessment. Food and Nutrition Sciences, 2(9): 989-1010.
Compound concentration data for PlantLIBRA cases
PlantLIBRA WP5 (HYLO) collected data about compound concentrations in three case study plants. The methods for the data collection are described elsewhere.
- Cinnamon (dried bark)
- Bitter fennel (fruit)
- Gingko (leaf)
The data were uploaded to Opasnet database. The database is a versatile data storage for all kinds of data tables related to benefit-risk assessment, health, food, and other topics. It has two parts: an open part that can be accessed using a web browser or through a machine-readable JSON interface; and a password-protected part that is technically similar but is not visible to the Internet.
Data on composition were collected running a systematic search on Pubmed, using information available from previous assessments of authoritative bodies (e.g. EFSA, EMA, etc.)and the ePlantLIBRA database, which was developed in this project. ePlantLIBRA is based on eBASIS from the EuroFIR Network. [1] eBASIS was produced by the EuroFIR network and is now being further developed in a co-operation between the PlantLIBRA Project and EuroFIR AISBL. EuroFIR is funded by the European Commission under the Food Quality and Safety Priority of the FP7 for Research and Technological Development (CT).
eBASIS is a unique database that contains critically evaluated published data on the content and biological effects of bioactive constituents in plant based foods and an up-to-date list of plant and plant part names in 15 EU languages. Bioactive Compounds are defined as inherent non-nutrient constituents of food plants and edible mushrooms with anticipated health promoting, beneficial and/or toxic effects when ingested. [2]
In an early stage there was a plan to store the compound data only in the ePlantlibra database and access that dynamically through a machine-readable interface from Opasnet. However, this plan was abandoned for several reasons. The main reason was that ePlantlibra database is actually only a part of the larger eBASIS database, which contains also proprietary, non-public information. It would have been a large work to build an additional security functionality to allow access to some but not all parts of the database. In addition, the tool we are describing here needs a moderate amount of data, which can easily be copied and uploaded to another system (Opasnet database) for this purpose. And thirdly, when new compound concentration data becomes available (either from ePlantlibra or elsewhere), it is straightforward to upload also the new data to Opasnet database as a part of Compound intake estimator. Thus, the use of Compound intake estimator is not dependent on ePlantlibra database but can benefit from its future development.
Case study results

 The following figures demonstrate the user interface and some output graphs from the model. The graphs give a user-friendly way to get an overview of the compounds in a product. It is especially interesting to compare concentrations and levels of concern of different compounds. As can be seen from the pair of graphs of cinnamon dried bark essential oil, most of the product is cinnamaldehyde, which is only marginally above a guidance value and thus not likely a concern. In contrast, safrole seems to be somewhat of concern or at least a compound to pay attention to, as it goes beyond a guidance value by a factor of 10 - 80.
The following figures demonstrate the user interface and some output graphs from the model. The graphs give a user-friendly way to get an overview of the compounds in a product. It is especially interesting to compare concentrations and levels of concern of different compounds. As can be seen from the pair of graphs of cinnamon dried bark essential oil, most of the product is cinnamaldehyde, which is only marginally above a guidance value and thus not likely a concern. In contrast, safrole seems to be somewhat of concern or at least a compound to pay attention to, as it goes beyond a guidance value by a factor of 10 - 80.

 Bitter fennel is a different story. The clearly largest concern is estragole, which goes beyond the BMDL10 by a factor of 50 - 600. Interestingly, it is at the same time one of the two main compounds in the product, implying that the concentration will most likely be high in all bitter fennel products. This product clearly needs a benefit-risk assessment, as the main compound may actually be causing a clear risk, raising the question about the use of this product in the first place. There is also another interesting compound, namely apiole, which goes above the level of concern by a factor of 10 - 200, even when its concentration is so low (0.1 %) that it is not even shown on the graph. With this compound, the first question in mind is whether there is actually evidence supporting its much higher putative potency compared with the other compounds.
Bitter fennel is a different story. The clearly largest concern is estragole, which goes beyond the BMDL10 by a factor of 50 - 600. Interestingly, it is at the same time one of the two main compounds in the product, implying that the concentration will most likely be high in all bitter fennel products. This product clearly needs a benefit-risk assessment, as the main compound may actually be causing a clear risk, raising the question about the use of this product in the first place. There is also another interesting compound, namely apiole, which goes above the level of concern by a factor of 10 - 200, even when its concentration is so low (0.1 %) that it is not even shown on the graph. With this compound, the first question in mind is whether there is actually evidence supporting its much higher putative potency compared with the other compounds.
Discussion
Concentration and intake data are not easily understood by consumers. There are dozens or hundreds of compounds in a single PFS product. It is therefore very difficult to get any idea which compounds are important and whether a consumer should be interested in further information about these compounds. There is a need for tools visualising these data to promote informed discussion and consumer decisions. The model presented here offers a solution to this need.
This model will make it easy to compare two critical things: exposure to compounds relative to health-based guidance values, and concentrations in the product. By comparing these two graphs, it was possible to identify from each example product two critical compounds that would warrant further scrutiny. The reasons for need of further work were different with each compound, as described in the previous section.
The compound intake estimator model can be used online simply with a web browser. It can easily be copied to other projects or uses in Opasnet. It can also be used on one's own computer, but that requires some skills of R software. It is a small task to add new data sets to the model when they become available. It is open source and thus it can be taken and developed further. It is a part of a large web workspace Opasnet that has several independent users and continuous, long-term maintenance. This flexibility and durability is important when considering the lifespan of the model. Even after the end of the PlantLIBRA project, the model will be maintained and kept available for users.
EFSA data collection and PFS
The critical thing with the compound intake estimator model is the availability of relevant compound concentration data about PFSs. EFSA has recently studied availability of such data. The EFSA contract CFT/EFSA/DCM/2011/03 provided EFSA with an updated food composition database covering approximately 1750 foods in combination with additional FoodEx2 facet descriptors included in the EFSA FoodEx2 classification system. A model for data transfer, compatible with the EuroFIR technical annex and CEN Food Data Standard, and the EFSA data structure was developed and tested. Fourteen national food database compiler organisations supplied initial food lists mapped to the EFSA food list. Eight of them contained data about food supplements and their composition. Datasets compatible with EFSA's data structure were produced based on the models. [3]
Therefore, there exists a large amount of potentially useful PFS compound concentration data in EFSA. However, EFSA has not made this data available for open use, and this will not probably happen before the end of PlantLIBRA project. In any case, it is important to acknowledge and remember this possibility and potential for improvement of the model described here.
| Country | Number of supplements listed |
|---|---|
| Finland | 92 |
| France | 449 |
| Italy | 126 |
| Netherlands | 902 |
| Serbia | 8 |
| Slovenia | 95 |
| Sweden | 122 |
| UK | 103 |
2Does not include supplements for children.
References
- ↑ ePlantlibra website, Eurofir, accessed 15th April, 2014.
- ↑ eBASIS (Bioactive Substances in Food Information System), Eurofir, accessed 15th April, 2014.
- ↑ 3.0 3.1 M.A. Roe, S. Bell, M. Oseredczuk, T. Christensen, S. Westenbrink, H. Pakkala, K. Presser and P.M. Finglas, 2013. Updated food composition database for nutrient intake. EFSA supporting publication 2013:EN-355, 21 pp. Available online: [4] [5] [6]