PCBs
This page is a encyclopedia article.
The page identifier is Op_en3461 |
|---|
| Moderator:Henrik (see all) |
|
|
| Upload data
|
Chemical structures
PCBs consist of 12 carbon atoms, forming two aromatic phenyl rings attached to one another through a carbon-carbon bridge, and 10 atoms that can be either hydrogens or chlorines (Figure 3). Theoretically 209 various combinations of chlorine and hydrogen are possible, and about 130 may be found in technical products. They are called congeners (see also ortho-PCBs). Chlorine increases the stability and decreases flammability of these compounds. The two phenyl rings of PCBs are able to rotate along the carbon-carbon bridge axis, and therefore they are flexible in the sense that they can assume a planar (flat) conformation similar to PCDDs, or a propeller-like conformation. ortho-Chlorines (in positions 2 and 6) may, however, prevent the planar conformation to a variable degree, and therefore ortho-congeners are less dioxin-like than non-ortho-congeners (see ortho-PCBs). Commercial PCBs contain PCDFs at levels up to 40 mg/kg, but usually not PCDDs.
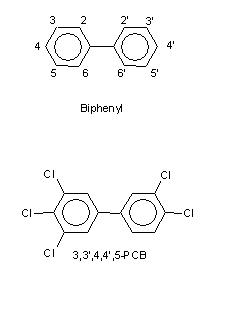 Figure 3. Structures of biphenyl and PCB.
Figure 3. Structures of biphenyl and PCB.
PCDDs consist of 12 carbon atoms, forming two aromatic phenyl rings attached to one another through two oxygen bridges, and 8 atoms that can be either hydrogens or chlorines (Figure 4). Theoretically 75 various combinations of chlorine and hydrogen are possible, and the resulting dibenzo-p-dioxin derivatives are called congeners (see this). Chlorine increases the stability of these compounds, and chlorines in positions 2,3,7, and 8 (lateral chlorines) are especially important, because they are essential for toxicity and also prevention of enzymatic destruction of PCDDs. Therefore the 7 congeners with 2,3,7,8-structure are toxicologically the most relevant. All additional chlorines to 2,3,7,8-structure decrease toxicity, but the spectrum of adverse effects remains similar (see TEF). Tetra-, penta-, hexa-, hepta-, and octachloro-derivatives are often called TCDD, PeCDD, HxCDD, HpCDD and OCDD, respectively.
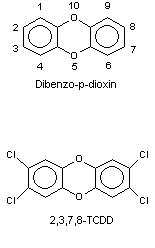 Figure 4. Structures of dibenzo-p-dioxin and TCDD.
Figure 4. Structures of dibenzo-p-dioxin and TCDD.
PCDFs consist of 12 carbon atoms, forming two aromatic phenyl rings attached to one another through one carbon-carbon bond and one oxygen bridge (Figure 5), and 8 atoms which can be either hydrogens or chlorines. Theoretically 135 various combinations of chlorine and hydrogen are possible, and the resulting dibenzofuran derivatives are called congeners. Chlorine increases the stability of these compounds, and chlorines in positions 2,3,7, and 8 (lateral chlorines) are especially important, because they increase toxicity and also prevent enzymatic destruction of PCDFs. Therefore the 10 congeners with 2,3,7,8-Cl-structure are toxicologically the most relevant. Most additional chlorines to 2,3,7,8-structure decrease toxicity (see TEF), but the spectrum of adverse effects remains similar. Tetra-, penta-, hexa-, hepta-, and octachloro-derivatives are often called TCDF, PeCDF, HxCDF, HpCDF and OCDF, respectively.
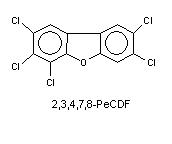 Figure 5. Structure of PeCDF.
[1]
Figure 5. Structure of PeCDF.
[1]