DARM DA study exercise Group 1
| Moderator:Anna Kokkonen (see all) |
| This page is a stub. You may improve it into a full page. |
| Upload data
|
Add a brief summary here.
Background
The 2009 flu pandemic was an outbreak of a new strain of H1N1 influenza virus, usually referred to as "swine flu". First described in April 2009, the influenza A(H1N1)v virus was a new virus subtype of influenza affecting humans, which contains segments of genes from pig, bird and human influenza viruses in a combination that had never been observed before anywhere in the world. A(H1N1)v virus is the result of a combination of two swine influenza viruses that contained genes of avian and human origin (ECDPC 2011). Unlike most strains of influenza, H1N1 does not disproportionately infect adults older than 60 years; this was an unusual and characteristic feature of the H1N1 pandemic (Writing Committee of the WHO 2010).
The outbreak began in the state of Veracruz, Mexico, with evidence that there had been an ongoing epidemic for months before it was officially recognized as such (McNeil 2009). The Mexican government closed most of Mexico City's public and private facilities in an attempt to contain the spread of the virus; however, it continued to spread globally, and clinics in some areas were overwhelmed by infected people. In June, the World Health Organization (WHO) and US Centers for Disease Control (CDC) stopped counting cases and declared the outbreak a pandemic (WHO/Chan 2009).
The H1N1 flu virus is typically contracted by person to person transmission through respiratory droplets (CDCP 2009). Symptoms usually last 4–6 days (Bronze 2009). The pandemic began to taper off in November 2009 (McKay 2009), and by May 2010, the number of cases was in steep decline (WHO 2010). On 10 August 2010 the World Health Organization announced the end of the H1N1 pandemic (Helsingin Sanomat 2010). According to the WHO statistics from July 2010, the virus had killed more than 18,000 people since it appeared in April 2009 (redOrbit 2010), approximately 4% of the 250,000 to 500,000 annual influenza deaths (WHO 2009).
Now the H1N1 influenza virus has moved into the post-pandemic period. However, localized outbreaks of various magnitudes are likely to continue. Influenza outbreaks, including those primarily caused by the H1N1 virus, show an intensity similar to that seen during seasonal epidemics. Recently published studies indicate that 20–40% of populations in some areas have been infected by the H1N1 virus and thus have some level of protective immunity. Many countries report good vaccination coverage, especially in high-risk groups, and this coverage further increases community-wide immunity (WHO 2010b).
In Finland the first laboratory confirmed cases of influenza A(H1N1) was discovered on May 2009. At that time the spread of influenza A(H1N1) to Finland was expected as the virus has spread widely around the world (THL 2009a). The first death in Finland associated with the A(H1N1)v influenza virus was confirmed in on October 2009. As of 26 October 2009, there had been 522 confirmed cases of influenza A(H1N1)v in Finland. In the same time the epidemic had continued its spread throughout Europe (THL 2009b).
The Ministry of Social Affairs and Health (MSAH) and the National Institute for Health and Welfare (THL) both recommended the vaccine, especially to the priority groups (health care professionals, pregnant women and persons aged from 6 months to 64 years belonging to a risk group due to another illness), to prevent the spread and severe complications of the illness (THL 2009b). The MSAH asked the THL to give opinion about getting vaccines. As the THL saw it, getting vaccines were very reasoned in that situation. By procurement it was confirmed to have vaccines fast. Firm reservation was made at the end of April 2009 right after the news from new epidemic had come (THL 2010).
Vaccination of health care personnel against influenza A(H1N1)v was initiated around the country during autumn. The plan was to start vaccinating pregnant women after health care personnel. After this, local authorities began vaccinating risk groups, according to the stated schedule. The proposal for the order of vaccination in Finland was approved by the Government in September 2009. The order was determined on medical grounds. Finland was among the first countries in Europe to receive the vaccine (THL 2009b).
The influenza vaccine being used in Finland was approved by the European Medicines Agency (EMEA), and it was also recommended by the World Health Organisation. The vaccine contained an adjuvant, a substance that enhances the immune response so less extract of the virus is needed in each dose. This immune response-enhancing substance had been thoroughly reviewed and tested even before it was considered for use in vaccines. The pandemic influenza vaccine had not been used in practice, but previous vaccines had provided much data and knowledge on the behaviour of parts of it and other closely related vaccines. THL announced that the vaccine might cause side effects similar to seasonal flu vaccines, like a sore arm from the shot, headache, muscles aches, joint pains and mild fever. The vaccine effectiveness was expected to be good, about 90 percent (THL 2009b).
Scope
Purpose
* Purpose defines the specific information need of the decision-making and the research question that is asked.
- purpose of the DA study
- question(s) addressed in the study
- the relation of the study to the overall swine flu case
- roles of different actors related to the study
- expected and possible impacts of the study
- intended (even if imaginary) use of the study
Boundaries
* Boundaries define which parts of the reality are taken into the assessment and which are excluded within
spatial, temporal and other dimensions.
- spatial and temporal boundaries the study
Scenarios
* Scenarios define particular conditions that are of interest irrespective whether they describe
reality or not (e.g. what-if scenarios).
Intended users
* Intended users are those for whom the assessment is made.
Participants
* Participants are those who may participate in the making of the assessment.
The minimum group of people for a successful assessment is always described.
If some groups must be excluded, this must be explicitly motivated.relevant actors related to the study
Definition
Upload a causal diagram and change the right name here.
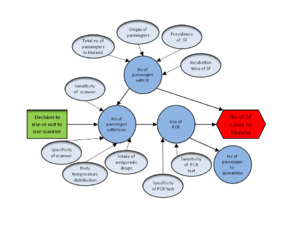
Decision variables
* Decision variables: decisions that are considered.
Indicators
* Indicators: outcome variables of interest.
Value variables
* Value variables: value judgements (usually about indicators).
Other variables
* Other variables: any variables that link to the causal network and are within the boundaries of the assessment.
Analyses
* Analyses: statistical and other analyses that contain two or more variables, e.g. optimizing.
Indices
* Indices: lists of particular locations along spatial, temporal, or other dimensions.
Result
* Results of indicators and assessment-specific analyses.
Results
Conclusions
* Conclusions are based on the results, given the scope.
See also
References
Chan M. 2009. World now at the start of 2009 influenza pandemic. World Health Organization.http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html. Published 11.6.2009
Centers for Disease Control and Prevention (CDCP). 2009. Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season. H1N1 Flu. http://www.cdc.gov/h1n1flu/recommendations.htm. Published 7.12.2009
Bronze, MS. H1N1 Influenza (Swine Flu). eMedicine. Medscape. http://emedicine.medscape.com/article/1673658-overview. Published 13.11.2009
Helsingin Sanomat. 2010. WHO julisti: Sikainfluenssa on ohi. Published 10.8.2010 http://www.hs.fi/kotimaa/artikkeli/WHO+julisti+Sikainfluenssa+on+ohi/1135259234155
McKay B. (2010-03-02). The Flu Season That Fizzled. The Wall Street Journal. http://online.wsj.com/article/SB10001424052748703429304575095743102260012.html. Published 2.3.2010
McNeil DG. 2009. In New Theory, Swine Flu Started in Asia, Not Mexico". The New York Times. http://www.nytimes.com/2009/06/24/health/24flu.html. Published 23.6.2009
National Institute for Health and Welfare (THL). 2009a. Ministry of Social Affairs and Health: Two cases of influenza A(H1N1) confirmed in Finland. Published 12.5.2009 http://www.thl.fi/en_US/web/en/pressrelease?id=13307
National Institute for Health and Welfare (THL). 2009b. THL and MSAH: Influenza A(H1N1)v epidemic about to start, first death in Finland confirmed. Published 27.10.2009 http://www.thl.fi/en_US/web/en/pressrelease?id=21364
National Institute for Health and Welfare (THL). 2010. Pandemiarokotehankinnasta piti päättää nopeasti. Published 19.11.2010 http://www.thl.fi/fi_FI/web/fi/uutinen?id=23508
RedOrbit. 2010. H1N1 Still A Pandemic, Says WHO. 2010. http://www.redorbit.com/news/health/1893907/h1n1_still_a_pandemic_says_who/. Published 20.7.2010
WHO. 2009. Influenza (Seasonal). April 2009. http://www.who.int/mediacentre/factsheets/fs211/en/. Retrieved 2010-02-13.
WHO. 2010a. Global Update on 2009 H1N1. Global Intensity Map, Week 17 (April 26, 2010-May 2, 2010). http://gamapserver.who.int/h1n1/qualitative_indicators/atlas.html?indicator=i2&date=Week%2017%20(26-Apr-2010%20:%2002-May-2010)
WHO 2010b. H1N1 in post-pandemic period. Director-General's opening statement at virtual press conference. Published 10.8.2010 http://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/index.html
Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. 2010. Clinical Aspects of Pandemic 2009 Influenza A (H1N1) Virus Infection. The New England Journal of Medicine 362: 1708–19 http://www.nejm.org/doi/full/10.1056/NEJMra1000449