PCDD/F
This page is a encyclopedia article.
The page identifier is Op_en3540 |
|---|
| Moderator:Henrik (see all) |
|
|
| Upload data
|
PCDD/F, an abbreviation for PCDDs and PCDFs.
Acute toxicity
This is quite variable in different animal species (Table 1). Lethal dose of TCDD to guinea pigs is about 0.001 mg/kg b.w., and to Rhesus monkeys 0.07 mg/kg b.w., but hamsters can tolerate over 1 mg/kg b.w. Even strains within the same species can show a similarly wide difference: the LD50 values for rats vary from 0.010 to >10 mg/kg. Human lethal dose is not known, but human beings are not assumed to be one of the most sensitive species. In Seveso accident, the highest human TCDD concentrations were 56000 ng/kg (in fat), and it can be estimated that the acute dose had been around 0.005 mg/kg b.w. No humans died in the accident, but lots of small animals such as rabbits were found dead at the accident area. No dose will kill the animal immediately, but a high dose causes the so called "wasting syndrome", the animal is anorectic and eats less than a quarter of the normal food intake, and dies after two to three weeks when the body weight has decreased by about 30-40 %. In some animals there may be liver damage including porphyria (disturbance of the synthesis of heme, the pigment of haemoglobin). Other typical features are atrophy of the thymus, disturbances in the levels of some amino acids and lipids, and induction of many oxidative enzymes. Other PCDD/Fs induce similar toxic effects, but they are less potent in line with their TEF (For detailed information, see Pohjanvirta & Tuomisto, Pharmacol. Rev. 1994:46:483-549). See also PCDD/F - toxicity in animals.[1]
| Species (strain) | LD50 (mg/kg) |
|---|---|
| Lake trout sack fry | 0.000074 |
| Guinea pig | 0.002 |
| Zebra fish sack fry | 0.0025 |
| Rat (Long-Evans) | 0.018 |
| Chicken | <0.025 |
| Rat (Sprague-Dawley) | 0.06 |
| Rabbit | 0.115 |
| Mouse (C57BL/6) | 0.182 |
| Mouse (DBA/2) | 2.57 |
| Hamster | >3 |
| Rat (Han/Wistar) | >10 |
Analysis
At the concentrations present in the environment or in living tissues, PCDD/Fs can be reliably analysed only by using Gas chromatography-mass spectrometry with high resolution. This is an expensive method and because of extensive sample purification steps, the procedure will take several weeks. There are few laboratories in Europe to assay PCDD/Fs reliably from animal or human tissues.[1]
Biomagnification
Property of PCDD/F compounds to concentrate from one trophic level to the next (see also Biomagnification). Many PCDD/Fs are extremely persistent in the environment. Increase in chlorination (see PCDD/F - physicochemical properties) increases both stability and lipophilicity. Therefore they concentrate along the food chain, and species at the "top" of the food chain (such as seals or eagles) are in special danger.[1]
Concentration in humans
Dioxins in the body are almost exclusively in fat because of their lipid solubility and poor water solubility. In some tissues dioxins may also be bound to specific proteins. The most reliable method to measure dioxin levels is to measure their concentrations in fat. Dioxin levels are the same on fat basis in most organs of the body, so there is plenty in very fatty tissues and little in lean tissues. Importantly there is the same concentration of dioxins in milk fat as in the fat of serum or of adipose tissue. This gives a possibility to measure dioxin levels without invasive methodology. WHO has organised two rounds of international intercalibrations whereupon milk dioxin levels in many countries were measured by strictly similar methods to make them comparable. The levels of seventeen PCDD/Fs in milk fat of primiparae mothers in central Europe were in 1994 about 20 ng/kg (as WHO TEqs in fat), and in less industrialised areas often around 10 ng/kg. However, since the cumulation of dioxins is very slow, the concentrations will increase during most of the lifetime. In Finland, the level in 20 year old population is 5-20 ng/kg (TEq in fat), but in 60 year old population it is 20-100 ng/kg. In chemical industries concentrations of up to several thousand ng/kg have been measured, and the highest measured concentrations in Seveso accident were 56,000 ng/kg (TCDD in fat). Dioxin concentrations have decreased during 1980s and 1990s (see also Body burden and PCDD/F - sources).[1]
Elimination
Process of discharging PCDD/Fs out of the body. This is very slow in all mammals, because these compounds are lipophilic, and cannot be excreted in urine, and also poorly degradable by the enzymatic machinery of the body (see also PCB - elimination). Generally, PCDFs are eliminated a little faster than PCDDs. Half lives of the 17 most important PCDD/Fs are shown in Table 2. (modified from Liem & Theelen, Dioxins: Chemical analysis, exposure and risk assessment, University of Utrecht, 1997).[1]
| Congener | |
| 2,3,7,8-TCDD | 6 – 10 |
| 1,2,3,7,8-PeCDD | 9 – 16 |
| 1,2,3,4,7,8-HxCDD | 8 |
| 1,2,3,6,7,8-HxCDD | 13 – >70 |
| 1,2,3,7,8,9-HxCDD | 5 – 9 |
| 1,2,3,4,6,7,8-HpCDD | 3 – 7 |
| OCDD | 6 – 7 |
| 2,3,7,8-TCDF | 0 – 4 |
| 1,2,3,7,8-PeCDF | 0.9 |
| 2,3,4,7,8-PeCDF | 5 – 20 |
| 1,2,3,4,7,8-HxCDF | 3 – 6 |
| 1,2,3,6,7,8-HxCDF | 4 – 6 |
| 1,2,3,7,8,9-HxCDF | ? |
| 2,3,4,6,7,8-HxCDF | 2 – 6 |
| 1,2,3,4,6,7,8-HpCDF | 3 – 7 |
| 1,2,3,4,7,8,9-HpCDF | 3 |
| OCDF | 0.2 – 2 |
Environmental persistence
Ability of PCDD/Fs to continue existence in the environment. Many PCDD/Fs are extremely persistent in the environment. Increase in chlorination (see PCDD/F - physicochemical properties) increases both stability and lipophilicity. Neither soil microbes nor animals are able to break down effectively those PCDDs with "lateral" chlorines, i.e. chlorines in positions 2,3,7, and 8. This causes especially slow elimination (see PCDD/F - elimination), and due to biomagnification (see also Bioaccumulation) those particular compounds are present in the organisms of higher trophic levels (such as birds and mammals). Since the same group of PCDDs with chlorines in 2,3,7,8-positions are also more toxic than others, they are toxicologically the most important congeners.[1]
Half-life
Time needed to decrease the amount of chemical to one-half (see PCDD/F - elimination).[1]
Limit values
Concentrations that are not to be exceeded in a matrix. There are no limit values in EU concerning human food. As to animal feed, there is a limit value for citrus pulp pellets: 500 pg/kg or 0.5 ng/kg (WHO-TEq in d.w.); for exhaust gases, there is a limit value of 1 ng/Nm3 (I-TEq in normalised cubic meter). Generally applied proposed values for PCDD/F in milk are as follows: target value <0.9 ng/kg (TEq in milk fat), limit value of distribution to consumers 3.0 ng/kg; ban for marketing 5.0 ng/kg.[1]
Physicochemical properties
All PCDD/Fs are non-volatile, lipophilic (soluble in fats and oils) and poorly water soluble, but lipophilicity increases by increasing rate of chlorination (see PCDD - chemical structure, PCDF - chemical structure). Their octanol/water distribution (indicating relative lipid to water solubility, see lipophilicity) is of the order of a million to hundred million (log Pow 6.5 to 8.8) explaining the high tendency to move toward lipids from water. [1]
Risk assessment
Yhis has been very difficult for several reasons. Mechanisms of toxicity are not yet understood. Large variations of acute toxicity between laboratory species have added to uncertainties in species extrapolation. Also variations in elimination rate are remarkable between species, half-life in rats is three weeks, in humans 7-8 years. Therefore long-term effects are more difficult to evaluate than short-term effects. Until recently cancer risk was considered the most important risk, and there was uncertainty and disagreement of the dose extrapolation. Recent epidemiological studies after high occupational and accidental exposures have, however, shown the cancer risk to be rather small, and in the general population it is probably not the most relevant risk. If there is any risk at the present background levels, it is likely to be developmental effects. One of the most sensitive effects seems to be the mineralization defect in teeth of children with the highest exposure via breast milk. WHO scientific panel reassessed dioxin risks in 1998, and the new recommendation for tolerable daily intake level is 1 to 4 pg/kg/day (in TEq per b.w.) during lifetime exposure. Two points are important here. One is that some of the most important sources are beneficial for health for other reasons, e.g. breast milk and fish. It is not rational to limit their use on the basis of theoretical risks. The second is that PCDD/Fs accumulate very slowly (see Cumulation). Therefore only exposures over years are important, unless the exposure is very high (such as an accident).[1]
Sources
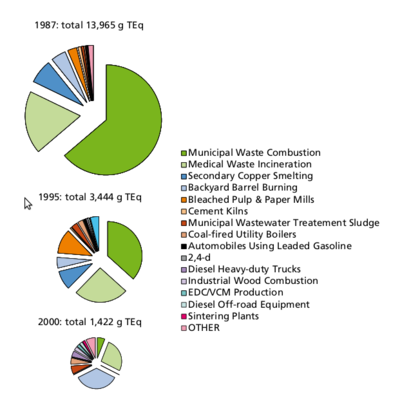
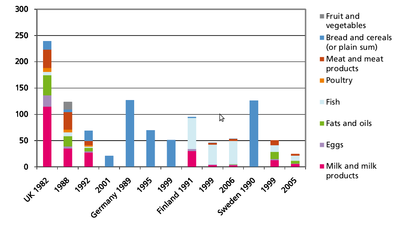
Formation: PCDD/Fs will form in small amounts, if there are carbon, oxygen and chlorine available in the presence of metal catalysts at suitable temperatures, which optimally means 400 to 700 °C. The most important sources of PCDD/Fs are incineration of mixed waste in too low temperatures, metal smelting and refining, chlorine bleaching of pulp (Figure 6). Another source is occurrence as impurities of many chlorinated chemicals such as PCBs, chlorophenols, phenoxy acid herbicides, and hexachlorophene. Human exposure. Food is the major source for human exposure to PCBs and dioxins, especially fatty foods: dairy products (butter, cheese, fatty milk), meat, egg, and fish (Figure 7). The current average body burden of dioxins is about 30-60 ng/kg (I-TEq in fat) or 300-600 ng (I-TEq per person) (see <a _fcknotitle="true" href="Body burden">Body burden</a>) which is close to the lowest concentrations possibly causing health effects. Average daily intake in many countries is 1 to 2 pg/kg (TEq per b.w.) or about 100 pg per person. Some subgroups within the society (e.g., nursing babies and people consuming plenty of fish) may be highly exposed to these compounds and are thus at greater risk. Dioxin concentrations have been screened in two WHO international studies, and in Central Europe the concentrations have decreased in breast milk from 30-40 ng/kg (TEq in milk fat) to 15-20 ng/kg from 1987 to 1993. The decrease in environmental concentrations is mostly due to improved incineration technology. [1]
Tooth effects
These were clearly shown in Yusho incident. At lower concentrations in Finland, defective mineralization of the first permanent molar teeth, which are formed during the two first years of life, correlated with PCDD/F exposure during breast-feeding. This may be the most sensitive effect of PCDD/Fs ever seen in humans.[1]
Toxicity in animals
Dioxins bring about a wide spectrum of biochemical and toxic effects in experimental animals. These effects depend on dose, species, strain, gender, age and tissue. Various dioxin congeners tend to elicit a similar battery of alterations, although the congeners are differently potent. TCDD serves as a surrogate for the whole group of chemicals. For the most part, the mechanisms of these impacts are still obscure. This hampers rational risk assessment. A common denominator appears to be the so called Ah-receptor, which mediates the biological effects of TCDD in cells. Some of the most toxic PCBs have dioxin-like toxicity based on AH receptor, but e.g. some effects on the nervous system are believed to have a different mechanism. A characteristic feature of the acute toxicity is an exceptionally large variation in sensitivity among species (see PCDD/F - acute toxicity). To the guinea pig, TCDD is the most toxic synthetic compound known with an LD50 value (dose lethal to 50% of animals) of only ca. 0.001 mg/kg, but the hamster tolerates a thousandfold higher dose. The reasons for these intra- and interspecies differences are unclear, but some are due to differences in the AH receptors. One of the most sensitive targets for TCDD appears to be the reproductive organ system in the developing foetus (Table 3). (For detailed information, see Pohjanvirta & Tuomisto, Pharmacol. Rev. 1994:46:483-549).[1]
Table 3. Some toxic and biochemical effects after TCDD, and body burdens related to the effects. Some of the data are based on the results of a single study.[1]
| Effect | Species | Body burden (ng/kg b.w.) |
|---|---|---|
| Adverse (toxic) effects | ||
| Immunological (viral sensitivity) | Mouse | 10 (LOEL) |
| Developmental neurotoxicity (object learning) | Rhesus monkey | 42 (LOEL) (maternal) |
| Reproductive toxicity (decreased sperm count) | Rat | 64 (LOEL) (foetal) |
| Hormonal (endometriosis) | Rhesus monkey | 69 (LOEL) |
| Chloracne | Human | 95 – 3000 |
| Tumour promotion | Rat | 2500 |
| Thyroid hormone (T4) decrease | Rat | 3000 (ED50) |
| Immunotoxicity (thymus atrophy) | Rat | 5000 (ED50) |
| Wasting syndrome | Rat | 5000 (ED50) |
| Biochemical effects | ||
| EGF receptor induction | Rat | 3 (LOEL) |
| IL1beta expression increase | Mouse | 10 (LOEL) |
| CYP1A1 enzyme induction | Mouse | 23 (LOEL) |
| CYP1A1 enzyme induction | Rat | 300 (ED50) |
LOEL: lowest observed effect level; ED50: median effective dose (causes 50 % of maximum effect). Data from e.g. WHO-ECEH/IPCS, 1998; DeVito et al., Environ Health Persp 103: 820-831, 1995.
Toxicity in humans
Acute toxicity after large doses was best seen after the <a _fcknotitle="true" href="Seveso accident">Seveso accident</a>. The most remarkable effect was chloracne, especially in children exposed to high doses appearing between two weeks and two months, and sometimes continuing for years. Chloracne has regularly been described even after heavy occupational exposures to PCDDs and other chlorinated chemicals. Also elevations of liver enzymes in blood were seen in Seveso victims indicating liver damage. There were signs of disturbed porphyrin metabolism (synthesis of heme, the pigment of haemoglobin) and increases in serum lipids (both triglycerides and cholesterol). A number of other health effects have been linked to high exposure to dioxins, including mood alterations, reduced cognitive performance, diabetes, changes in white blood cells, dental defects, endometriosis, decreased male/female ratio of births and decreased testosterone and (in neonates) elevated thyroxin levels. As yet such effects have not been proven as caused by PCDD/Fs. The effect that has caused the greatest public concern is cancer, and IARC recently classified TCDD as a human carcinogen (see Polychlorinated dibenzo-p-dioxin). Another concern in the society are the possible developmental effects. There is recent data that dioxin exposure from breast milk is associated with abnormal development and mineralization of teeth.[1]
Use
PCDD/Fs have never been synthesised for any other purpose than research, but they are formed as unintentional by-products in chemical syntheses of 2,4,5-trichlorophenol (an intermediate of antiseptic hexachlorophene and herbicide 2,4,5-T) and in many burning processes (see PCDD - sources).[1]
References
</references>
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 Jouko Tuomisto, Terttu Vartiainen and Jouni T. Tuomisto: Dioxin synopsis. Report. National Institute for Health and Welfare (THL), ISSN 1798-0089 ; 14/2011 [1]
- ↑ King, K. Compilation of EU Dioxin Exposure and Health Data, Task 4 - Human Exposure. AEA Technology 1999, [2]; Kiviranta H. Exposure and human PCDD/F and PCB body burden in Finland, Publicat. Natl. Public Hlth Inst. A14/2005, [3]; Törnkvist A. et al. Chemosphere 2011:83:193, [4].