Epidemiological modelling: Difference between revisions
No edit summary |
No edit summary |
||
| Line 176: | Line 176: | ||
</rcode> | </rcode> | ||
=== | === Initializing the functions === | ||
<rcode name="alusta" label=" | <rcode name="alusta" label="Initialize functions" embed=1> | ||
library(OpasnetUtils) | library(OpasnetUtils) | ||
Revision as of 13:28, 24 June 2014
| Moderator:Nobody (see all) Click here to sign up. |
|
|
| Upload data
|
Question
How to predict the net effectiveness of a pneumococcal conjugate vaccination with a given set of serotypes when the vaccine is included in the national immunisation programme?
- Focus is on the number of invasive pneumococcal disease (IPD) cases in different age groups.
- The model is assumed to be valid in a population in which an infant pneumococcal conjugate vaccination has been in use for several years s.t. a new steady-state after vaccination has been reached. Coverage of vaccination and vaccine efficacy against carriage are assumed to be high enough to justify the assumtion of full elimination of vaccine type carriage among both the vaccinated and also, due to substancial herd effects, among the unvaccinated members of the population.
- Vaccine type carriage is fully replaced by carriage of the non-vaccine types and the disease causing potential of different serotypes is not altered by vaccination.
Answer
Predicted number of invasive pneumococcal disease (IPD) cases in different age groups are obtained from the serotype replacement model (Nurhonen and Auranen, 2014).
Rationale
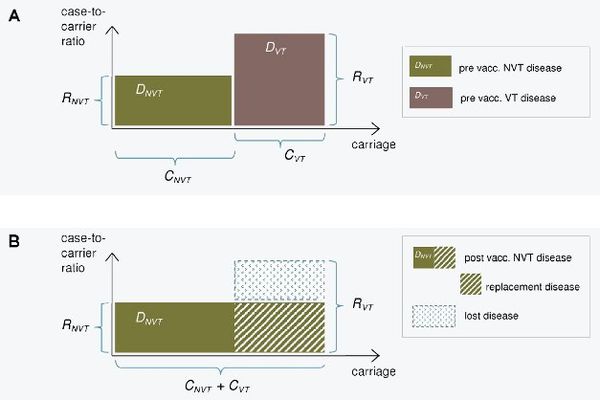
The epidemiological model for pneumococcal carriage and disease is based on the assumption that vaccination completely eliminates the vaccine type carriage in a vaccinated population and this carriage is replaced by non-vaccine type carriage. The implications of this replacement on the decrease or increse in pneumococcal disease then depend on the disease causing potential of the replacing types compared to that of the replaced types. To predict post vaccination disease only pre vaccination data on serotype specific carriage and disease is used.
The consequences of serotype replacement in the model depend on two key assumptions regarding the new steady-state after vaccination:
- the relative serotype proportions among the non-vaccine types are not affected by vaccination (proportionality assumption);
- the case-to-carrier ratios (the disease causing potentials) of individual serotypes remain at their pre-vaccination levels.
The implications of vaccination on disease incidence are assumed to be solely due to the elimination of vaccine type carriage and its replacement by non vaccine type carriage. An exception to this is when a possibility of efficacy against disease without any efficacy against carriage is assumed for certain serotypes.
Computation
The following program (modified from File S1 in Nurhonen and Auranen, 2014) illustrates the working of the replacement model. In its current implementation the code asks the user to choose a vaccine composition (labelled "New") and then displays the predicted IPD cases in Finland per year corresponding to this vaccine. The results are shown by serotype and by age category (<5 and 5+ year olds) and the corresponding results for PCV10 (labelled "Current") are displayed for comparison.
Instructions for user: Choose the desired vaccine composition from the list below and then press "Run code". The results will be displayed on the right side of the page. The default choice is PCV10 and this should be changed either by adding or removing serotypes as PCv10 is the vaccine to which the new vaccine is compared. For PCV13, add serotypes 6A, 19A and 3.
Initializing the functions
See also