Uncertainty in health risks due to anthropogenic primary fine particulate matter from different source types in Finland: Difference between revisions
m (Created page with "{{nugget |authors=M. Tainio, Jouni T. Tuomisto, J. Pekkanen, N. Karvosenoja, K. Kupiainen, P. Porvari, M. Sofiev, A. Karppinen, L. Kangas, J. Kukkonen |reference= ...") |
|||
| (28 intermediate revisions by the same user not shown) | |||
| Line 180: | Line 180: | ||
presented in Fig. 1. Different modules that are part of the integrated | presented in Fig. 1. Different modules that are part of the integrated | ||
assessment model are described in the following chapters. | assessment model are described in the following chapters. | ||
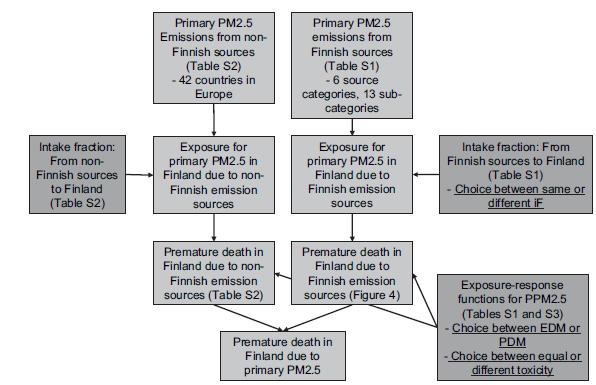
[[Image:Unc1.JPG|thumb|center|650px|'''Fig. 1.''' A schematic flowchart of the integrated assessment model. The arrows denote a flow of information, and the rectangles are the sub-models and the intermediate or final | |||
results. Model uncertainties associated with different parts of the model are underlined. (PDM - Performance-based decision maker, EDM - Equal-weight decision maker).]] | |||
===Emissions=== | ===Emissions=== | ||
| Line 269: | Line 272: | ||
primary PM2.5 matter in Finland from: | primary PM2.5 matter in Finland from: | ||
<math> | <math> | ||
C=(Q\ | C=(Q\times iF)/(Pop\times Br)</math> | ||
The primary PM2.5 emission strengths (Q) for Finnish emissions | The primary PM2.5 emission strengths (Q) for Finnish emissions | ||
| Line 325: | Line 328: | ||
material) and for primary PM2.5 emissions from other European | material) and for primary PM2.5 emissions from other European | ||
countries, we used the average exposure-response function from | countries, we used the average exposure-response function from | ||
the expert elicitation study. The sensitivity of the model in the | the expert elicitation study. The sensitivity of the model in the toxicity assumptions was tested in the sensitivity analysis (see | ||
below). | |||
===Health effects=== | |||
The premature death due to chronic exposure to primary PM2.5 | |||
was estimated with the equation: | |||
D=C\times Cr\times B | |||
where D is premature non-accidental death in 2000 due to PM2.5 | |||
exposure, C is the concentration increase of pollutant due to | |||
a specified emission source category, Cr is percentage change | |||
in non-accidental mortality due to permanent 1 mg m�3 change | |||
in PM2.5 exposure and B background non-accidental mortality in | |||
Finland in 2000. | |||
Background mortality statistics for Finland for 2000 were | |||
obtained from World Health Organization (WHO) Mortality Database | |||
(http://www.who.int/healthinfo/morttables/). The non-accidental | |||
mortality was calculated by subtracting accidental causes | |||
(International Classification of Disease (ICD) version 10 codes V01- | |||
Y89) from total mortality. The background hazard rates for one year | |||
were estimated based on age categories of the WHO mortality | |||
database and for United Nation World Population Prospects: The | |||
2006 Revision and World Urbanization Prospects population data | |||
for 2000 (UN, 2008). | |||
The loss of life-expectancy attributable to primary PM2.5 exposure | |||
was estimated with the life-table model described in Tainio | |||
et al. (2007). Based on sensitivity analyses performed in Tainio | |||
et al. (2007), we omitted the time lag calculations from the | |||
model. In this case, lag is defined as the time elapsing between | |||
a change in exposure and the ensuing change in the hazard rate. | |||
The premature death was estimated for all emission source categories | |||
separately and the life-expectancy to all sources combined. | |||
===Sensitivity analysis=== | |||
The sensitivity analysis for input variables was performed by | |||
calculating absolute rank-order correlations between input and | |||
output variables. This sensitivity analysis method is called importance | |||
analysis. Throughout the model, both parameter and model | |||
uncertainties were evaluated based on the literature or author | |||
judgment. Parameter uncertainties contain those related to the | |||
input variables, such as the uncertainty of the annual emissions of | |||
primary PM2.5 originating from domestic wood combustion. The | |||
model uncertainties contain those caused by the physical and | |||
chemical limitations of the models, such as using the same or | |||
source category specific exposure-response functions in the model. | |||
The uncertainties for emissions and exposure estimates are presented | |||
in Tables S1eS3 (Supplementary material). All the uncertainties | |||
were assumed to be independent of each other (thus, | |||
uncorrelated). | |||
Both parameter and model uncertainties were propagated | |||
through the model by Monte Carlo simulation. The model uncertainty | |||
was described with binary variables (Bernoulli distribution), | |||
choosing between two alternative model branches. Parameter | |||
uncertainty was described by using continuous distributions. The | |||
effects of parameter and model uncertainties on model results were | |||
studied using importance analysis. The importance analysis was | |||
undertaken by calculating absolute rank-order correlations | |||
between the input variables and the model results. The model was | |||
implemented using Analytica � version 4.2. (Lumina Decision | |||
Systems, Inc., CA) Monte Carlo simulation program and run with | |||
50 000 iterations. | |||
==Results== | |||
The primary PM2.5 emissions from Finnish and European | |||
anthropogenic sources, addressed in this study, were estimated be | |||
responsible for 209 (mean, 95% CI 6-739) and 357 (mean, 95% CI 8- | |||
1482) premature deaths, respectively, in the population of Finland | |||
in 2000 and to lower the average life-expectancy by 0.12 (mean, | |||
95% CI 0.00e0.48) years. The average exposures of Finnish population | |||
to primary PM2.5 were estimated to be 0.33 (mean, 95% CI | |||
0.18e0.55) due to Finnish and 0.65 <math>\mu mg^{-3}</math> (mean, 95% CI | |||
0.34e1.08) due to European sources. | |||
===Finnish emission sources=== | |||
The premature deaths due to primary PM2.5 emissions from | |||
Finland were estimated taking account both emission-exposure | |||
and toxicity differences, and model uncertainties. Fig. 2 illustrates | |||
how the emission-exposure and toxicity variations between | |||
primary PM2.5 emission source categories affected the relative | |||
importance of these sources. The relative importance of trafficoriginated | |||
primary PM2.5 emissions increased from approximately | |||
30% (emissions) to 50% (premature deaths) when both emissionexposure | |||
and toxicity variations were taken into account. | |||
Table 1 describes how the mean premature death estimates | |||
changed between source categories when emission-exposure, | |||
toxicity variation or both were taken into account. The premature | |||
deaths due to traffic emission were elevated by 240% and premature | |||
deaths due to agriculture emissions decreased by 70% in | |||
comparison to premature death estimates without source category | |||
specification. For the other source categories, the impact of emission- | |||
exposure and toxicity variation was smaller (Fig. 2, Table 1). | |||
The exposure-response function uncertainty was the main | |||
uncertainty in the integrated assessment with over 90% correlation | |||
between the exposure-response function uncertainty and the | |||
uncertainty of the premature death estimate (Fig. 3). The choice | |||
between equal or emission source specific exposure-response | |||
functions was the second most important uncertainty for those | |||
sub-categories for which we assumed differential toxicity. The | |||
main exposure uncertainty was the ability of the dispersion models | |||
to predict primary PM2.5 concentrations. The emission uncertainties | |||
had the greatest importance for source categories ‘other | |||
sources’, ‘domestic combustion’ and ‘small power plants’ (Fig. 3). | |||
The emission source category ‘traffic’ contributed almost a half | |||
(85 premature deaths annually) of all the adverse health effects due | |||
to Finnish primary PM2.5 emissions in Finland (Fig. 4). The second | |||
most important emission source category was ‘domestic combustion’ | |||
(45 premature deaths) and the third most important was | |||
‘power plants’ (29 premature deaths). These emission source | |||
categories accounted for most of the adverse health effects, | |||
regardless of the model assumptions (Table 1). | |||
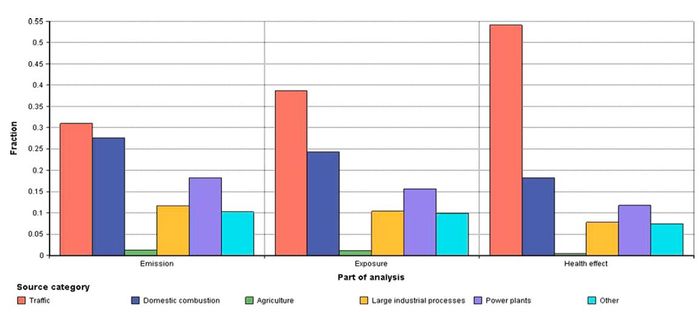
[[Image:Unc2.JPG|thumb|center|700px|'''Fig. 2.''' The fractions of emissions, exposure and health effects in Finland due to six Finnish primary PM2.5 emission source categories. For example, emission source category ‘Traffic’ | |||
causes approximately 30% of emissions, 40% of exposure and 50% of adverse health effects.]] | |||
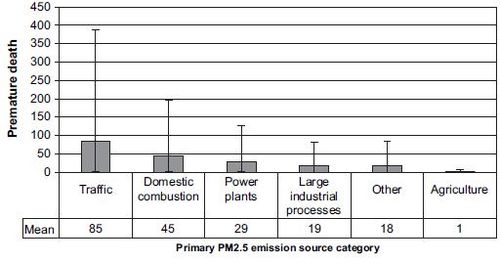
[[Image:Unc4.JPG|thumb|center|500px|'''Fig. 4.''' The premature deaths in Finland in 2000 due to anthropogenic primary PM2.5 | |||
emissions from Finland (mean and 95% CI). The premature death estimates have been | |||
estimated separately for six emission source categories. The emission source categories | |||
are described in Table S1 (Supplementary material). The premature death in Finland | |||
due to other European primary PM2.5 emissions is presented in Table S2 | |||
(Supplementary material).]] | |||
{|{{prettytable}} | |||
|+'''Table 1.''' Comparison of mean premature death estimates for different primary PM2.5 emission source categories without and with source category specific emission-exposure (iF) and exposure-response (toxicity) variations. (iF = intake fraction). | |||
|- | |||
|----- | |||
! | |||
!colspan=4|Premature death | |||
!colspan=3|Comparison in relation to model without source specific iF or toxicity | |||
|----- | |||
| | |||
|Without source specific iF or toxicity | |||
|Source specific iF specific iF or toxicity | |||
|Source specific toxicity | |||
|Source specific iF and toxicity | |||
|Source specific iF | |||
|Source specific toxicity | |||
|Source specific iF and toxicity | |||
|----- | |||
|Traffic | |||
|55 | |||
|72 | |||
|102 | |||
|133 | |||
|130% | |||
|185% | |||
|240% | |||
|----- | |||
|Domestic combustion | |||
|50 | |||
|45 | |||
|50 | |||
|45 | |||
|92% | |||
|100% | |||
|92% | |||
|----- | |||
|Agriculture | |||
|2 | |||
|2 | |||
|1 | |||
|1 | |||
|94% | |||
|30% | |||
|28% | |||
|----- | |||
|Large industrial processes | |||
|21 | |||
|19 | |||
|21 | |||
|19 | |||
|92% | |||
|100% | |||
|92% | |||
|----- | |||
|Power plants | |||
|33 | |||
|29 | |||
|33 | |||
|29 | |||
|89% | |||
|100% | |||
|89% | |||
|----- | |||
|Other | |||
|19 | |||
|19 | |||
|19 | |||
|19 | |||
|100% | |||
|100% | |||
|100% | |||
|----- | |||
|Sum | |||
|179 | |||
|186 | |||
|225 | |||
|246 | |||
|<nowiki>-</nowiki> | |||
|<nowiki>-</nowiki> | |||
|<nowiki>-</nowiki> | |||
|} | |||
===Other European sources=== | |||
Over half of the premature deaths due to the exposure to | |||
primary PM2.5 in Finland in 2000 were estimated to be due to | |||
primary PM2.5 emissions outside Finland (Table S2, Supplementary | |||
material). The primary PM2.5 emissions from the neighboring | |||
countries of Russia, Sweden, and Estonia explained most of the | |||
adverse health effects. Additionally, approximately 34 premature | |||
deaths were associated with the primary PM2.5 emissions from | |||
Ukraine. | |||
These substantial contributions of the neighboring countries | |||
and Ukraine are caused (i) on one hand by the relatively high iF | |||
values (these are associated both with the geographic proximity | |||
and the location of those countries with respect to the prevailing wind circulation patterns) and (ii) on the other hand by the relatively | |||
high emission strengths (in case of Russia and Ukraine). The | |||
premature death estimates due to European sources were sensitive | |||
to exposure-response function uncertainties (data not shown). | |||
==Discussion== | |||
===Source category variation=== | |||
We have developed an integrated assessment model to evaluate | |||
the premature deaths caused by primary PM2.5 from different | |||
source categories. Fig. 2 and Table 1 present how the emissionexposure | |||
and toxicity variation between source categories | |||
substantially increased the relative importance of source category | |||
‘traffic’ and decreased the relative importance of source category | |||
‘agriculture’. Reliable information about relative contribution of | |||
different emission sources to population health is important in | |||
selecting the most effective mitigation actions. | |||
The emission-exposure variation between different emission | |||
source categories has been studied rarely. Wolff (2000) concluded | |||
that taking into account the emission-exposure variations, the | |||
rank-ordering of available mitigation actions changed in comparison | |||
to mass based comparison. Fann et al. (2009) concluded that | |||
a PM emission reduction is subject to high variation, depending on | |||
the location of the emissions in U.S. In this study we have also | |||
revealed that both emission-exposure and toxicity variation will | |||
affect the results of integrated assessment and consequently the | |||
rank-ordering of available mitigation actions. | |||
There were only small differences for the emission-exposure | |||
relationships between various primary PM2.5 source categories. | |||
The emission-exposure relationships used in this study were based | |||
on the results of a regional scale dispersion model with approximately | |||
5 km spatial resolution over Finland (Tainio et al., 2009). As | |||
discussed in that study, the finite resolution of the dispersion | |||
model leads to an underestimation of the exposure, especially for | |||
sources with low emission heights near to population hotspots. For | |||
example, Wolff (2000) estimated a 4-fold difference in population | |||
average iF between traffic and power plant emissions, while in this | |||
study the difference was 1.4-fold. Thus, the iFs evaluated by Tainio | |||
et al. (2009) tend to underestimate the emission-exposure relationships, | |||
especially for traffic and possibly for domestic combustion | |||
primary PM2.5 emissions, since emissions from these sources | |||
are released from low emission heights and in most cases, in | |||
relatively densely populated areas. This indicates that the emissionexposure | |||
variations between sources may be potentially even | |||
higher than those presented in this study, and that the emissionexposure | |||
variation could well have even greater impacts on the | |||
results of the integrated assessments. | |||
The toxicity variation had major impacts on premature death | |||
estimates (Table 1). The exposure-response functions for different | |||
primary PM2.5 emission source categories were based on experts’ | |||
estimates of the most and least toxic substances in the PM2.5 mass | |||
(Cooke et al., 2007; Tuomisto et al., 2008). The substances that | |||
experts named as the least and the most toxic varied between the | |||
experts and therefore the combined estimate (that was used in this | |||
study) represents the maximum variation between the least and | |||
the most toxic substance in PM2.5 mass. Clearly, there are inherent | |||
uncertainties in using this kind of expert based approach, and the | |||
results for different emission source categories should be viewed | |||
more as indicative rather than quantitative. | |||
Previous assessments have only rarely estimated toxicity | |||
differences between different particles, although several epidemiological | |||
studies have detected toxicity differences between PM | |||
from different sources (e.g. Laden et al., 2000; Lanki et al., 2006). In | |||
the European Externalities of Energy (ExternE) study, primary PM | |||
from traffic was evaluated to be 1.5 times more toxic than the | |||
average PM2.5 mass, while secondary sulfate and nitrate had | |||
a lower toxicity than the average PM2.5 mass (ExternE, 2005). The | |||
2005 update of ExternE methodology did not include a sensitivity | |||
analysis and the impact of these toxicity variations in the assessment | |||
was not evaluated. A recent study on regional background | |||
exposure for PM2.5 in Europe used a 2.8 times higher RR estimate | |||
for primary PM2.5 mass in comparison to secondary PM2.5 mass | |||
(Andersson et al., 2009). They concluded that in Europe the exposure | |||
for secondary PM2.5 is higher than exposure for primary PM2.5 | |||
but due to toxicity differences, the magnitude of adverse health | |||
effects was similar. | |||
This study was based on toxicity variation between different | |||
emission source categories. However, the toxicity is not dependent | |||
per se on the emission sources but rather on the chemical and | |||
physical properties of the inhaled PM. In an ideal case the exposureresponse | |||
functions would be based on the chemical and physical | |||
properties of inhaled PM. However, the current knowledge on the | |||
adverse health effects of PM2.5 is inadequate to permit this kind of | |||
exposure-response modeling. | |||
===Uncertainties=== | |||
The uncertainties related to exposure-response functions were | |||
identified to possess the highest importance in the health effect | |||
estimates (Fig. 3). A similar result has been noted in our previous | |||
assessment studies for PM2.5 air pollution (Leino et al., 2008; Tainio | |||
et al., 2005, 2007) and in a number of other assessment studies (e.g. | |||
Künzli et al., 2000; Levy and Spengler, 2002). | |||
The uncertainties related to input variables and for the model | |||
structure were evaluated with various methods. In a few cases, the | |||
uncertainty was based on previously published studies (e.g. emission | |||
uncertainties). For some of the input variables the uncertainty | |||
was defined with expert estimate (e.g. exposure-response functions). | |||
With respect to the other variables the uncertainty was | |||
estimated based on author judgment. Thus, different uncertainties | |||
were defined with different methods ranking from the expert study | |||
to the crude guess of the modeler. This raises a doubt of the | |||
comparativeness of different uncertainty estimates in the sensitivity | |||
analysis. However, the purpose of this study was more to | |||
demonstrate the model and to define those uncertainties that | |||
require a more formal analysis in future assessments than provide | |||
formal uncertainty assessment. | |||
We assumed in this study much higher uncertainty to exposureresponse | |||
functions than previous assessment studies. For example, | |||
in the Pope et al., 2002 study, a <math>1 \mu g m^{-3}</math> change in PM2.5 concentration | |||
was estimated to change non-accidental mortality between | |||
0.15% and 1.00% (95% confidence interval). In this study, the corresponding | |||
variationwas 0.03e4.57% (for average exposure-response function). When testing the sensitivity of the model for different | |||
uncertainties by using similar uncertainty intervals as in Pope et al. | |||
(2002), the relative importance of exposure-response function | |||
uncertainties was still most important but only with small differences | |||
e.g. compared to the importance of the uncertainty in the | |||
dispersion model (data not presented). Thus, exposure-response | |||
function uncertainty remained important also while using less | |||
uncertain input variables. | |||
We also assumed in this study that all the uncertainties are | |||
independent from each other. Thus, we did not assume any correlation | |||
between uncertainties. This could result in either under- or | |||
overestimation of total uncertainty of the model. However, we | |||
assume that this would not change significantly the results of this | |||
study since we compared mainly uncertainties that are uncorrelated | |||
(e.g. toxicity to iF). | |||
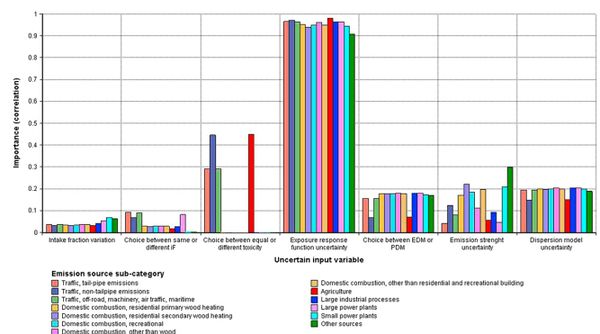
[[Image:Unc3.JPG|thumb|center|600px|'''Fig. 3.''' The importance analysis results. The figure shows rankeorder correlation between seven input variables and the modeled premature deaths in Finland due to Finnish primary PM2.5 emissions in 2000 for the 13 emission source sub-categories. A high correlation indicates that the input variable has a strong impact on the model output. (iF - intake fraction, ER - Exposure-response, PDM - Performance-based decision maker, EDM - Equal-weight decision maker).]] | |||
===Magnitude of health effects=== | |||
The primary PM2.5 originating from the whole of Europe were | |||
estimated to cause in Finland approximately 566 premature deaths | |||
and to lower the average life expectancy by 0.12 years in 2000. The | |||
CAFE program has evaluated that PM2.5 were responsible for the | |||
premature deaths of 1270 Finns (Watkiss et al., 2005). When one | |||
takes into account that the CAFE study included both primary and | |||
secondary PM2.5, the results between CAFE and this study are not in | |||
disagreement. Over half of the adverse health effects in this study | |||
were due to long-range transported primary PM2.5 from other | |||
European countries. The exposure for local emission sources is | |||
probably underestimated in this study due to reasons discussed | |||
earlier. | |||
We estimated that traffic-originated primary PM2.5 emissions | |||
from Finland caused approximately 85 premature deaths in Finland | |||
in 2000. In our previous studies, we have estimated that primary | |||
PM2.5 from local buses in Helsinki region would be responsible for | |||
18 premature deaths in 2020 (Tainio et al., 2005) and that the | |||
primary PM2.5 due to the heavy-duty fleet (including buses) | |||
account for 34 premature deaths per year in the Helsinki metropolitan | |||
area (Leino et al., 2008). In our previous studies, the | |||
exposure was based on personal measurements of PM2.5 and the | |||
sources were identified with the source apportionment method. | |||
The comparison of these two studies therefore indicate that this | |||
study may underestimate the health effects for traffic-originated | |||
primary PM2.5, most probably due to underestimated exposure. | |||
The magnitude of underestimation in exposure can also be | |||
estimated by comparing the measured PM concentrations to | |||
modeled ones. In this study the average population exposure in | |||
Finland for all primary PM2.5 emission sources included in the | |||
computations combined was less than 1<math>\mu g m^{-3}</math>. For comparison, in | |||
2001 the average PM2.5 concentrations, including secondary PM, in | |||
Helsinki region varied between 8<math>\mu g m^{-3}</math> and 9<math>\mu g m^{-3}</math> at two | |||
measurement stations (YTV, 2002). Although Helsinki is more | |||
polluted than the rest of the Finland, a substantial fraction of the | |||
measured PM2.5 are secondary PM, and present study did not | |||
include non-anthropogenic sources, less than 1<math>\mu g m^{-3}</math> average | |||
exposure seems an underestimation of exposure. | |||
The Finnish anthropogenic primary PM2.5 emissions were estimated | |||
in this study to be 30.8 (95% CI 27e36) Gg/a for the year | |||
2000. In the European Thematic Strategy on Air Pollution assessment, | |||
in the year 2000 the primary PM2.5 emissions for Finland | |||
were 28 Gg/a based on the RAINS model (Amann et al., 2006). The | |||
higher emission strength in this study in comparison to RAINS is | |||
due to different emission source definitions of the traffic non-tailpipe | |||
emissions (RAINS do not include traffic induced dust | |||
suspension). Another emission inventory maintained by European | |||
Monitoring and Evaluation Programme (EMEP) and based on | |||
country submissions of national inventories estimated 38.2 Gg/ | |||
a primary PM2.5 emissions for Finland for 2000 (Vestreng et al., | |||
2006). The higher value is mainly due to the outdated estimate of | |||
domestic wood combustion emissions in the EMEP database | |||
(Karvosenoja, 2008). | |||
We assumed no threshold for the primary PM2.5 induced | |||
premature deaths. The previous assessments have shown that | |||
a threshold value can have major impact on the results of the | |||
assessment (e.g. Künzli et al., 2000). There have been several statistical | |||
attempts to define a threshold for PM2.5 air pollution. For | |||
example, Schwartz et al. (2002, 2008) have studied nonlinearities in | |||
exposure-response functions in order to define a threshold for shortterm( | |||
acute) and long-term (chronic) effect of PM2.5, respectively, but | |||
neither of these studies detected any threshold value for PM2.5. The | |||
World Health Organization working group stated in 2003 that | |||
epidemiological studies have been unable to identify any threshold | |||
for PM2.5 and that it is likely that the PM2.5 is harmful in the population | |||
since all populations contain susceptible individuals (WHO, | |||
2003). However, in the expert elicitation study conducted by U.S. | |||
Environmental Protection Agency (EPA), a number of experts gave | |||
separate exposure-response coefficients and/or plausibility for the | |||
PM2.5 air pollution mortality impact of low exposure levels (usually | |||
below 10<math>\mu g m^{-3}</math>) (Roman et al., 2008). Whether or not there is any | |||
threshold remains one of the important undefined uncertainties. | |||
==Conclusions== | |||
We have utilized an integrated assessment model to estimate | |||
the adverse health effects mainly due to anthropogenic primary | |||
fine particulate matter originating from different emission source | |||
categories. The variations in both emission-exposure and toxicities | |||
between source categories had significant impacts for the assessment | |||
results, especially for traffic-originated primary fine particulate | |||
matter. This kind of information is important for the rank | |||
ordering of the effectiveness of the available mitigation actions. The | |||
main uncertainties in the model were related to exposure-response | |||
functions and to the estimation of emission-exposure relationships | |||
for different source categories. | |||
==Acknowledgements== | |||
This study was done as a part of the projects KOPRA (funded by | |||
the Ministry of the Environment, Finland, Grant YM119/481/2002, | |||
the National Technology Agency of Finland (Tekes), Grant 616/31/ | |||
02 and the Helsinki Metropolitan Area Council (YTV), Grant 135/ | |||
03), BIOHER (funded by the Academy of Finland, Grant 10155) and | |||
PILTTI (funded by the Ministry of the Environment, Finland, Grant | |||
YM57/065/2005). The work relates to projects INTARESE (funded | |||
by European Union, Grant 018385-2), MEGAPOLI (funded by | |||
European Union) and SCUD (funded by the Academy of Finland, | |||
Grant 111775). Marko Tainio was supported by personal grant from | |||
the Graduate School in Environmental Health. We would like to | |||
thank Dr. Ewen MacDonald for checking the English language. | |||
==Appendix. Supplementary material== | |||
Supplementary data associated with this article can be found in | |||
the online version at {{doi|10.1016/j.atmosenv.2010.02.036}}. | |||
==References== | |||
Amann, M., Asman, W., Bertok, I., Cofala, J., Heyes, J., Klimont, Z., Posch, M., | |||
Schöpp, W., Wagner, F., Hettelingh, J.-P., 2006. Emission control scenarios that | |||
meet the environmental objectives of the thematic strategy on air pollution. | |||
Laxenburg, Austria: NEC scenario analysis report Nr. 2. International Institute | |||
for Applied Systems Analysis (IIASA). | |||
Andersson, C., Bergström, R., Johansson, C., 2009. Population exposure and | |||
mortality due to regional background PM in Europe long-term simulations of | |||
source-region and shipping contributions. Atmospheric Environment 43, | |||
3614-3620. | |||
Bennett, D.H., McKone, T.E., Evans, J.S., Nazaroff, W.W., Margni, M.D., Jolliet, O., | |||
Smith, K.R., 2002. Defining intake fraction. Environmental Science & Technology | |||
36, 206a-211a. | |||
Cooke, R.M., Wilson, A.M., Tuomisto, J.T., Morales, O., Tainio, M., Evans, J.S., 2007. A | |||
Probabilistic characterization of the relationship between fine particulate | |||
matter and mortality: elicitation of European experts. Environmental Science & | |||
Technology 41, 6598-6605. | |||
ExternE - Externalities of Energy - Methodology 2005 Update. Luxembourg: Office | |||
for Official Publications of the European Communities. Available at: http://www.externe.info/brussels/methup05a.pdf. | |||
Fann, N., Fulcher, C.M., Hubbell, B.J., 2009. The influence of location, source, and | |||
emission type in estimates of the human health benefits of reducing a ton of air | |||
pollution. Air Quality, Atmosphere, and Health 2, 169-176. | |||
Hopke, P.K., Ito, K., Mar, T., Christensen, W.F., Eatough, D.J., Henry, R.C., Kim, E., | |||
Laden, F., Lall, R., Larson, T.V., Liu, H., Neas, L., Pinto, J., Stolzel, M., Suh, H., | |||
Paatero, P., Thurston, G.D., 2006. PM source apportionment and health effects: | |||
1. Intercomparison of source apportionment results. Journal of Exposure | |||
Science & Environmental Epidemiology 16, 275-286. | |||
Karvosenoja, N., 2008. Emission Scenario Model for Regional Air Pollution. Monographs | |||
of the Boreal Environment Research No. 32. Available at: http://www.ymparisto.fi/download.asp?contentid¼92446&lan¼en. | |||
Karvosenoja, N., Tainio, M., Kupiainen, K., Tuomisto, J.T., Kukkonen, J., Johansson, M., | |||
2008. Evaluation of the emissions and uncertainties of PM2.5 originated from | |||
vehicular traffic and domestic wood combustion in Finland. Boreal Environment | |||
Research 13, 465-474. | |||
Künzli, N., Kaiser, R., Medina, S., Studnicka, M., Chanel, O., Filliger, P., Herry, M., | |||
Horak, F., Puybonnieux-Texier, V., Quenel, P., Schneider, J., Seethaler, R., | |||
Vergnaud, J.C., Sommer, H., 2000. Public-health impact of outdoor and trafficrelated | |||
air pollution: a European assessment. Lancet 356, 795-801. | |||
Kupiainen, K.J., Karvosenoja, N., Porvari, P., Johansson, M., Tainio, M., Tuomisto, J.T., | |||
2006. Emissions of primary carbonaceous particles, their uncertainties and | |||
spatial allocation in Finland. In proceedings from the IUAPPA regional conference/ | |||
17th EFCA speciality conference, September 6-8, 2006, Lille, France. | |||
Laden, F., Neas, L.M., Dockery, D.W., Schwartz, J., 2000. Association of fine particulate | |||
matter from different sources with daily mortality in six US cities. Environmental | |||
Health Perspectives 108, 941-947. | |||
Lanki, T., de Hartog, J.J., Heinrich, J., Hoek, G., Janssen, N.A.H., Peters, A., Stolzel, M., | |||
Timonen, K.L., Vallius, M., Vanninen, E., Pekkanen, J., 2006. Can we identify | |||
sources of fine particles responsible for exercise-induced ischemia on days with | |||
elevated air pollution? The ULTRA study. Environmental Health Perspectives | |||
114, 655-660. | |||
Leino, O., Tainio, M., Tuomisto, J.T., 2008. Comparative risk analysis of dioxins in fish | |||
and fine particles from heavy-duty vehicles. Risk Analysis 28, 127-140. | |||
Levy, J.I., Spengler, J.D., 2002. Modeling the benefits of power plant emission | |||
controls in Massachusetts. Journal of the Air & Waste Management Association | |||
52, 5-18. | |||
Pope, C.A., Burnett, R.T., Thun, M.J., Calle, E.E., Krewski, D., Ito, K., Thurston, G.D., | |||
2002. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine | |||
particulate air pollution. JAMA - Journal of the American Medical Association | |||
287, 1132-1141. | |||
Pope, C.A., Dockery, D.W., 2006. Health effects of fine particulate air pollution: lines | |||
that connect. Journal of the Air & Waste Management Association 56, 709-742. | |||
Roman, H.A., Walker, K.D., Walsh, T.L., Conner, L., Richmond, H.M., Hubbell, B.J., | |||
Kinney, P.L., 2008. Expert judgment assessment of the mortality impact of | |||
changes in ambient fine particulate matter in the US. Environmental Science & | |||
Technology 42, 2268-2274. | |||
Schwartz, J., Laden, F., Zanobetti, A., 2002. The concentration-response relation | |||
between PM2.5 and daily deaths. Environmental Health Perspectives 110, | |||
1025-1029. | |||
Schwartz, J., Coull, B., Laden, F., Ryan, L., 2008. The effect of dose and timing of dose | |||
on the association between airborne particles and survival. Environmental | |||
Health Perspectives 116, 64-69. | |||
Tainio, M., Tuomisto, J.T., Hänninen, O., Aarnio, P., Jantunen, M., Pekkanen, J., 2005. | |||
Health effects caused by primary fine particulate matter (PM2.5) emitted from | |||
busses in Helsinki metropolitan area, Finland. Risk Analysis 25, 151-160. | |||
Tainio, M., Tuomisto, J.T., Hänninen, O., Ruuskanen, J., Jantunen, M.J., Pekkanen, J., | |||
2007. Parameter and model uncertainty in a life-table model for fine particles | |||
(PM2.5): a statistical modeling study. Environmental Health: a Global Access | |||
Science Source 6. | |||
Tainio, M., Sofiev, M., Hujo, M., Tuomisto, J.T., Loh, M., Jantunen, M.J., Karppinen, A., | |||
Kangas, L., Karvosenoja, N., Kupiainen, K., Porvari, P., Kukkonen, J., 2009. Evaluation | |||
of the intake fractions of primary fine particulate matter originated from | |||
various source categories. Atmospheric Environment 43, 1962-1971. | |||
Tuomisto, J.T., Wilson, A., Evans, J.S., Tainio, M., 2008. Uncertainty in mortality | |||
response to airborne fine particulate matter: combining European air pollution | |||
experts. Reliability Engineering & System Safety 93, 732-744. | |||
UN (United Nation), 2008. Population division of the department of economic and | |||
social affairs of the United Nations Secretariat, world population prospects: the | |||
2006 revision and world urbanization prospects: the 2005 revision Available at: | |||
http://esa.un.org/unpp (accessed 06.02.03). | |||
Vestreng, V., Rigler, E., Adams, M., Kindbom, K., Pacyna, J.M., Denier van der Gon, H., | |||
Reis, S., Travnikov, O., 2006. Inventory review 2006. Emission data reported to | |||
the LRTAP Convention and NEC Directive: stage 1, 2 and 3 review, and evaluation | |||
of inventories of HMs and POPs. MSC-W Technical report 1/2006. | |||
Available at: http://emep.int/publ/reports/2006/emep_technical_1_2006.pdf. | |||
Watkiss, P., Pye, S., Holland, M., 2005. Baseline Scenarios for Service Contract for | |||
carrying out cost-benefit analysis of air quality related issues, in particular in | |||
the clean air for Europe (CAFE) programme. AEAT/ED51014/ Baseline Issue 5. | |||
WHO (World Health Organization), 2003. Health aspects of air pollution with | |||
particulate matter, ozone and nitrogen dioxide. Report on a WHO working | |||
group. Bonn, Germany 13e15 January 2003. Available at: http://www.euro.who.int/document/e79097.pdf. | |||
WHO (World Health Organization), 2007. Health Relevance of Particulate Matter | |||
from Various Sources. Report on a WHO Workshop. WHO, Bonn, Germany. | |||
Wolff, S.K., 2000. Evaluation of Fine Particle Exposures, Health Risks and Control | |||
Options. Department of Environmental Health, Harvard School of Public Health, | |||
Boston, U.S. | |||
YTV (Helsinki Metropolitan Area Council), 2002. Ilmanlaatu pääkaupunkiseudulla | |||
vuonna 2001. (Air quality in Helsinki metropolitan area in 2001). Pääkaupunkiseudun | |||
Julkaisusarja C 17 (2002). | |||
Latest revision as of 14:49, 27 April 2012
This page is a nugget.
The page identifier is Op_en5563 | |
|---|---|
| Moderator:Essi Vuorinen (see all) | |
|
| |
| Upload data
|
Unlike most other pages in Opasnet, the nuggets have predetermined authors, and you cannot freely edit the contents. Note! If you want to protect the nugget you've created from unauthorized editing click here |
This page (including the files available for download at the bottom of this page) contains a draft version of a manuscript, whose final version is published and is available in the Atmospheric Environment 44 (2010). If referring to this text in scientific or other official papers, please refer to the published final version as: M. Tainio, J.T. Tuomisto, J. Pekkanen, N. Karvosenoja, K. Kupiainen, P. Porvari, M. Sofiev, A. Karppinen, L. Kangas, J. Kukkonen: Uncertainty in health risks due to anthropogenic primary fine particulate matter from different source types in Finland. Atmospheric Environment 44 (2010) 2125e2132 doi:10.1016/j.atmosenv.2010.02.036 .
Title
Editing Uncertainty in health risks due to anthropogenic primary fine particulate matter from different source types in Finland
Authors and contact information
- M. Tainio, correspondence author
- (Marko.Tainio@thl.fi)
- (Department of Environmental Health, National Institute for Health and Welfare (THL), Kuopio, Finland)
- (Systems Research Institute, Polish Academy of Sciences, Warsaw, Poland)
- J.T. Tuomisto
- (Department of Environmental Health, National Institute for Health and Welfare (THL), Kuopio, Finland)
- (Academy of Finland, Helsinki, Finland)
- J. Pekkanen
- (Department of Environmental Health, National Institute for Health and Welfare (THL), Kuopio, Finland)
- (University of Eastern Finland, Kuopio, Finland)
- N. Karvosenoja
- (Finnish Environment Institute (SYKE), Helsinki, Finland)
- K. Kupiainen
- (Finnish Environment Institute (SYKE), Helsinki, Finland)
- P. Porvari
- (Finnish Environment Institute (SYKE), Helsinki, Finland)
- M. Sofiev
- (Finnish Meteorological Institute (FMI), Helsinki, Finland)
- A. Karppinen
- (Finnish Meteorological Institute (FMI), Helsinki, Finland)
- L. Kangas
- (Finnish Meteorological Institute (FMI), Helsinki, Finland)
- J. Kukkonen
- (Finnish Meteorological Institute (FMI), Helsinki, Finland)
Article info
Article history:
Received 4 June 2009
Received in revised form 18 January 2010
Accepted 24 February 2010
Abstract
The emission-exposure and exposure-response (toxicity) relationships are different for different emission source categories of anthropogenic primary fine particulate matter (PM2.5). These variations have a potentially crucial importance in the integrated assessment, when determining cost-effective abatement strategies.We studied the importance of these variations by conducting a sensitivity analysis for an integrated assessment model. The model was developed to estimate the adverse health effects to the Finnish population attributable to primary PM2.5 emissions from the whole of Europe. The primary PM2.5 emissions in the whole of Europe and in more detail in Finland were evaluated using the inventory of the European Monitoring and Evaluation Programme (EMEP) and the Finnish Regional Emission Scenario model (FRES), respectively. The emission-exposure relationships for different primary PM2.5 emission source categories in Finland have been previously evaluated and these values incorporated as intake fractions into the integrated assessment model. The primary PM2.5 exposure-response functions and toxicity differences for the pollution originating from different source categories were estimated in an expert elicitation study performed by six European experts on air pollution health effects. The primary PM2.5 emissions from Finnish and other European sources were estimated for the population of Finland in 2000 to be responsible for 209 (mean, 95% confidence interval 6e739) and 357 (mean, 95% CI 8e1482) premature deaths, respectively. The inclusion of emission-exposure and toxicity variation into the model increased the predicted relative importance of traffic related primary PM2.5 emissions and correspondingly, decreased the predicted relative importance of other emission source categories.We conclude that the variations of emission-exposure relationship and toxicity between various source categories had significant impacts for the assessment on premature deaths caused by primary PM2.5.
Abbreviations
CAFE, Clean Air For Europe-program; CI, Confidence interval; EDM, Equal-weight decision maker; EMEP, European Monitoring and Evaluation Programme; EPA, U.S. Environmental Protection Agency; ER, Exposure-response; ExternE, Externalities of Energy; FRES, Finnish Regional Emission Scenario model; ICD, International Classification of Disease; iF, Intake fraction; PDM, Performancebased decision maker; PM, Particulate matter; PM2.5, Fine particulate matter; RAINS, Regional Air Pollution Information and Simulation e model; SILAM, Air Quality and Emergency Modeling System; WHO, World Health Organization.
Keywords
Fine particulate matter, Intake fraction, Exposure-response, Integrated assessment, Sensitivity analysis
Introduction
Integrated assessment models can describe quantitative dependences between emission sources and the adverse health effects. This is essential to understand these dependences if one wishes to devise effective emission mitigation strategies.
Fine particulate matter (PM2.5) air pollution has been associated with several adverse health effects (e.g. Pope and Dockery, 2006). The exposure to PM2.5 originating from different sources or source categories can be estimated with atmospheric dispersion models or with source apportionment of measured PM mass (Hopke et al., 2006). Most of the integrated assessment studies for PM are based on dispersion modeling. For example, Levy and Spengler (2002) demonstrated a modeling framework to estimate the adverse health effects due to secondary PM2.5 formed from precursor gas emissions from two power plants in Massachusetts, U.S. Künzli et al. (2000) estimated the exposure for traffic related PM in Austria, France and Switzerland. Both of the above mentioned studies have focused on one or a few emission sources, or a single emission source category.
The exposure assessment studies have rarely taken into account PM emissions from several source categories. One such study is the European Clean Air For Europe (CAFE) -program that evaluated the adverse health effects due to PM2.5 in Europe, taking into account all anthropogenic PM2.5 emission sources in Europe (Watkiss et al., 2005). However, the CAFE program did not report any source category specific results. Another study performed in the U.S. (Fann et al., 2009), evaluated the effectiveness of emission reduction of different air pollutants in 9 urban areas and for 5 emission source categories. They concluded that reduction of carbonaceous PM would generate the largest benefit in all locations, in comparison to secondary PM.
The assessment studies for PM2.5 usually assume equal toxicity for all PM2.5, regardless of the pollution source or chemical composition. Toxicity is here defined to refer to the ability of air pollution to cause adverse health effects to a human population. The chemical and physical properties of PM are known to be substantially different for various emission sources, and these properties could modify the relative toxicity of PM2.5. For example, several epidemiological studies have reported in both U.S. and Europe that one obtains higher relative risk estimates for PM from combustion sources (e.g., Laden et al., 2000; Lanki et al., 2006). However, there is limited understanding of the differences of toxicity in terms of PM properties. A World Health Organization (WHO) workshop concluded in 2007 that the current scientific knowledge is not sufficient to differentiate the toxicity of different PM sources (WHO, 2007). Despite the lack of information, the workshop acknowledged the need to perform sensitivity analyses for toxicity differences in integrated assessments.
In this study, we describe an integrated assessment model to estimate the adverse health effects due to anthropogenic primary PM2.5 from six different emission source categories, taking into account also the differences in emission-exposure relationships, and the variations of toxicity between PM from different source categories. In this study we did not take into account the formation of secondary PM2.5 from anthropogenic or natural gaseous emissions. We also did not include all the source categories of primary PM2.5 from natural emissions (for instance, wild-land fires and sea salt aerosols); however, fugitive dust was included.
The aims of this study were (i) to develop an integrated assessment model that allows an estimation of the adverse health effects caused by primary PM2.5, (ii) to estimate how emissionexposure and toxicity differences for various primary PM2.5 source categories influence the results of the assessments, (iii) to evaluate the uncertainties in emissions, dispersion, and health effects, and (iv) to estimate the primary PM2.5 induced premature deaths and the change in life-expectancy in Finland in 2000. The modeling of life-expectancy, the uncertainties of emissions, and emissionexposure relationships have been previously published in Tainio et al. (2007), Karvosenoja et al. (2008), and Tainio et al. (2009), respectively.
Materials and methods
The schematic flowchart of the integrated assessment model is presented in Fig. 1. Different modules that are part of the integrated assessment model are described in the following chapters.
Emissions
The emissions of anthropogenic primary PM2.5 from Finland and from other European countries in 2000 were estimated using two different emission datasets. The emissions of primary PM2.5 from Finland were estimated with the Finnish Regional Emission Scenario (FRES) model (Karvosenoja, 2008). The FRES model has calculated primary PM2.5 emissions with detailed chemical and size-segregation splits. The emissions are derived from 205 point sources with detailed plant and stack characteristics, and area sources with aggregation into 112 source categories and 15 fuels. In this study, the emissions of anthropogenic primary PM2.5 in Finland were divided into 6 emission source categories, which were further divided into 13 sub-categories. These source categories and subcategories are described in the supplementary material (Table S1).
The primary PM2.5 emission strength uncertainties for Finnish emissions were estimated for all 13 sub-categories. The primary PM2.5 emission strength uncertainties for traffic and domestic wood combustion have been evaluated by Karvosenoja et al. (2008). They concluded that the uncertainties of small-scale domestic combustion emissions were responsible for most of the emission uncertainties regarding primary PM2.5 from these two source categories. The emission model used by Karvosenoja et al. (2008) was used also in this study.
The primary PM2.5 emission strength uncertainties for other emission source categories were estimated separately for activities and emission factors, based on Karvosenoja (2008), Karvosenoja et al. (2008) and Kupiainen et al. (2006). Both the uncertainties of activities and emission factors were highest for emission source categories ‘small power plants’ and ‘other sources’. The emission strengths of annual average primary PM2.5 emissions from Finland in 2000 and their respective uncertainties are presented in the supplementary material (Table S1). Spatial uncertainty (i.e., the location of emissions, which is not known with accuracy) was not included in these uncertainties.
The emission strengths of anthropogenic primary PM2.5 for other European countries were derived from the European Monitoring and Evaluation Programme (EMEP) database (www.emep. int). The emission strengths were estimated for all anthropogenic sources combined in 2000 and separately for each of the 42 countries (Table S2, Supplementary material). Emission strength uncertainties for these emissions have not been quantitatively reported.
Dispersion and exposure
The population exposure for primary PM2.5 in Finland due to primary PM2.5 emissions from Finland and elsewhere in Europe was evaluated, and presented using the concept of intake fraction (Bennett et al., 2002). The intake fraction is defined as “integrated incremental intake of a pollutant released from a source category and summed over all exposed individuals” (Bennett et al., 2002). In this study, exposure estimates are based on outdoor concentration of primary PM2.5. The exposures in this study are based on values reported by Tainio et al. (2009); they calculated the emissionexposure relationships for various primary PM2.5 emission source categories in Europe using the iF methodology.
The atmospheric dispersion of primary PM2.5 was evaluated using the dual-core Lagrangian-Eulerian dispersion model SILAM (http://silam.fmi.fi), for the primary PM2.5 emissions in 2000. The dispersion was computed for two different study domains. In the European domain, the emissions of primary PM2.5 were based on EMEP data and the concentrations were estimated with approximately with a horizontal resolution of 30 km over the whole of Europe. In the Northern European domain (that included most of Scandinavia and some surrounding regions), the primary PM2.5 emission strengths for Finnish sources were based on the values provided by the FRES model and the concentrations were estimated approximately with a horizontal resolution of 5 km. In the Northern European domain, the PM2.5 concentrations were estimated separately for six different emission source categories. A constant breathing rate of 20 dm3 day�1 (w0.0002m3 s�1)was used in the iF calculations.
The iF values were evaluated in Tainio et al. (2009) using:
Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle iF=\sum(Ci*Pop_i)*Br/Q} ,
where Pop is the number of population (persons), C is the concentration increase of pollutant due to a specified emission source category or area of emissions (g m-3), Br is the breathing rate (m3/s/person), and Q is the emission rate (g s-1). In this study, we estimated the population-weighted average exposure for primary PM2.5 matter in Finland from: Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle C=(Q\times iF)/(Pop\times Br)}
The primary PM2.5 emission strengths (Q) for Finnish emissions were updated, in comparison to Tainio et al. (2009). For the primary PM2.5 emission strengths for other European sources, for the population (Pop) data and for the breathing rate (Br), we used the same values as reported in Tainio et al. (2009). The iF data for primary PM2.5 emissions originated from Finland and elsewhere in Europe are summarized in the supplementary material (Tables S1eS2), respectively, with other exposure uncertainties provided in Table S3.
Exposure-response functions
The exposure-response function describes the change in the background health effect attributable to the change in the exposure level. We used the term exposure-response function to emphasize that although the exposure was estimated based on outdoor concentrations of PM, the concentration is used as a proxy for exposure.
The exposure-response functions for primary PM2.5 mortality impacts were extracted from a formal elicitation of expert judgment performed for six European air pollution experts (Cooke et al., 2007; Tuomisto et al., 2008). In the expert elicitation study, experts provided quantitative estimates of mortality impacts of hypothetical short- and long-term changes in PM2.5 concentrations in US and Europe, and for several other variables. The expert answers were combined with two methods: with equal-weight between the experts (equal-weight decision maker, EDM) and with assessing weights based on seed questions on which answers were known (performance-based decision maker, PDM). The experts also gave their estimates for the least and most toxic element of the PM mixture and defined those elements. All the experts assumed that combustion PM were more toxic than the average PM2.5 mass (Cooke et al., 2007). Two experts assumed that traffic related combustion PM (referred as diesel or traffic PM) would be more toxic than the average PM2.5 mass. Four experts claimed that secondary PM (sulfate, nitrate or both) and two experts speculated that PM2.5 from crustal sources would be less toxic than the average PM2.5 mass. The uncertainties were recognized as being high.
For this study, we adopted exposure-response functions for primary PM2.5 induced non-accidental mortality, caused by longterm (chronic) exposure to PM, from the expert-elicitation study (Table S3). Two additional assumptions on toxicity were made. First, we assumed that all the primary PM2.5 possess equal toxicity, regardless of source. For this, we used an average exposureresponse function based on the expert elicitation study. Second, we assumed from the experts’ estimates that the most toxic substances originated from traffic tailpipe emissions (both for on-road and offroad vehicles and machinery) and the least toxic substances are from traffic non-tailpipe and agricultural emissions. For all the other emission source sub-categories (Table S1, Supplementary material) and for primary PM2.5 emissions from other European countries, we used the average exposure-response function from the expert elicitation study. The sensitivity of the model in the toxicity assumptions was tested in the sensitivity analysis (see below).
Health effects
The premature death due to chronic exposure to primary PM2.5 was estimated with the equation:
D=C\times Cr\times B
where D is premature non-accidental death in 2000 due to PM2.5 exposure, C is the concentration increase of pollutant due to a specified emission source category, Cr is percentage change in non-accidental mortality due to permanent 1 mg m�3 change in PM2.5 exposure and B background non-accidental mortality in Finland in 2000.
Background mortality statistics for Finland for 2000 were obtained from World Health Organization (WHO) Mortality Database (http://www.who.int/healthinfo/morttables/). The non-accidental mortality was calculated by subtracting accidental causes (International Classification of Disease (ICD) version 10 codes V01- Y89) from total mortality. The background hazard rates for one year were estimated based on age categories of the WHO mortality database and for United Nation World Population Prospects: The 2006 Revision and World Urbanization Prospects population data for 2000 (UN, 2008).
The loss of life-expectancy attributable to primary PM2.5 exposure was estimated with the life-table model described in Tainio et al. (2007). Based on sensitivity analyses performed in Tainio et al. (2007), we omitted the time lag calculations from the model. In this case, lag is defined as the time elapsing between a change in exposure and the ensuing change in the hazard rate. The premature death was estimated for all emission source categories separately and the life-expectancy to all sources combined.
Sensitivity analysis
The sensitivity analysis for input variables was performed by calculating absolute rank-order correlations between input and output variables. This sensitivity analysis method is called importance analysis. Throughout the model, both parameter and model uncertainties were evaluated based on the literature or author judgment. Parameter uncertainties contain those related to the input variables, such as the uncertainty of the annual emissions of primary PM2.5 originating from domestic wood combustion. The model uncertainties contain those caused by the physical and chemical limitations of the models, such as using the same or source category specific exposure-response functions in the model. The uncertainties for emissions and exposure estimates are presented in Tables S1eS3 (Supplementary material). All the uncertainties were assumed to be independent of each other (thus, uncorrelated).
Both parameter and model uncertainties were propagated through the model by Monte Carlo simulation. The model uncertainty was described with binary variables (Bernoulli distribution), choosing between two alternative model branches. Parameter uncertainty was described by using continuous distributions. The effects of parameter and model uncertainties on model results were studied using importance analysis. The importance analysis was undertaken by calculating absolute rank-order correlations between the input variables and the model results. The model was implemented using Analytica � version 4.2. (Lumina Decision Systems, Inc., CA) Monte Carlo simulation program and run with 50 000 iterations.
Results
The primary PM2.5 emissions from Finnish and European anthropogenic sources, addressed in this study, were estimated be responsible for 209 (mean, 95% CI 6-739) and 357 (mean, 95% CI 8- 1482) premature deaths, respectively, in the population of Finland in 2000 and to lower the average life-expectancy by 0.12 (mean, 95% CI 0.00e0.48) years. The average exposures of Finnish population to primary PM2.5 were estimated to be 0.33 (mean, 95% CI 0.18e0.55) due to Finnish and 0.65 Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu mg^{-3}} (mean, 95% CI 0.34e1.08) due to European sources.
Finnish emission sources
The premature deaths due to primary PM2.5 emissions from Finland were estimated taking account both emission-exposure and toxicity differences, and model uncertainties. Fig. 2 illustrates how the emission-exposure and toxicity variations between primary PM2.5 emission source categories affected the relative importance of these sources. The relative importance of trafficoriginated primary PM2.5 emissions increased from approximately 30% (emissions) to 50% (premature deaths) when both emissionexposure and toxicity variations were taken into account.
Table 1 describes how the mean premature death estimates changed between source categories when emission-exposure, toxicity variation or both were taken into account. The premature deaths due to traffic emission were elevated by 240% and premature deaths due to agriculture emissions decreased by 70% in comparison to premature death estimates without source category specification. For the other source categories, the impact of emission- exposure and toxicity variation was smaller (Fig. 2, Table 1).
The exposure-response function uncertainty was the main uncertainty in the integrated assessment with over 90% correlation between the exposure-response function uncertainty and the uncertainty of the premature death estimate (Fig. 3). The choice between equal or emission source specific exposure-response functions was the second most important uncertainty for those sub-categories for which we assumed differential toxicity. The main exposure uncertainty was the ability of the dispersion models to predict primary PM2.5 concentrations. The emission uncertainties had the greatest importance for source categories ‘other sources’, ‘domestic combustion’ and ‘small power plants’ (Fig. 3).
The emission source category ‘traffic’ contributed almost a half (85 premature deaths annually) of all the adverse health effects due to Finnish primary PM2.5 emissions in Finland (Fig. 4). The second most important emission source category was ‘domestic combustion’ (45 premature deaths) and the third most important was ‘power plants’ (29 premature deaths). These emission source categories accounted for most of the adverse health effects, regardless of the model assumptions (Table 1).
| Premature death | Comparison in relation to model without source specific iF or toxicity | ||||||
|---|---|---|---|---|---|---|---|
| Without source specific iF or toxicity | Source specific iF specific iF or toxicity | Source specific toxicity | Source specific iF and toxicity | Source specific iF | Source specific toxicity | Source specific iF and toxicity | |
| Traffic | 55 | 72 | 102 | 133 | 130% | 185% | 240% |
| Domestic combustion | 50 | 45 | 50 | 45 | 92% | 100% | 92% |
| Agriculture | 2 | 2 | 1 | 1 | 94% | 30% | 28% |
| Large industrial processes | 21 | 19 | 21 | 19 | 92% | 100% | 92% |
| Power plants | 33 | 29 | 33 | 29 | 89% | 100% | 89% |
| Other | 19 | 19 | 19 | 19 | 100% | 100% | 100% |
| Sum | 179 | 186 | 225 | 246 | - | - | - |
Other European sources
Over half of the premature deaths due to the exposure to primary PM2.5 in Finland in 2000 were estimated to be due to primary PM2.5 emissions outside Finland (Table S2, Supplementary material). The primary PM2.5 emissions from the neighboring countries of Russia, Sweden, and Estonia explained most of the adverse health effects. Additionally, approximately 34 premature deaths were associated with the primary PM2.5 emissions from Ukraine.
These substantial contributions of the neighboring countries and Ukraine are caused (i) on one hand by the relatively high iF values (these are associated both with the geographic proximity and the location of those countries with respect to the prevailing wind circulation patterns) and (ii) on the other hand by the relatively high emission strengths (in case of Russia and Ukraine). The premature death estimates due to European sources were sensitive to exposure-response function uncertainties (data not shown).
Discussion
Source category variation
We have developed an integrated assessment model to evaluate the premature deaths caused by primary PM2.5 from different source categories. Fig. 2 and Table 1 present how the emissionexposure and toxicity variation between source categories substantially increased the relative importance of source category ‘traffic’ and decreased the relative importance of source category ‘agriculture’. Reliable information about relative contribution of different emission sources to population health is important in selecting the most effective mitigation actions.
The emission-exposure variation between different emission source categories has been studied rarely. Wolff (2000) concluded that taking into account the emission-exposure variations, the rank-ordering of available mitigation actions changed in comparison to mass based comparison. Fann et al. (2009) concluded that a PM emission reduction is subject to high variation, depending on the location of the emissions in U.S. In this study we have also revealed that both emission-exposure and toxicity variation will affect the results of integrated assessment and consequently the rank-ordering of available mitigation actions.
There were only small differences for the emission-exposure relationships between various primary PM2.5 source categories. The emission-exposure relationships used in this study were based on the results of a regional scale dispersion model with approximately 5 km spatial resolution over Finland (Tainio et al., 2009). As discussed in that study, the finite resolution of the dispersion model leads to an underestimation of the exposure, especially for sources with low emission heights near to population hotspots. For example, Wolff (2000) estimated a 4-fold difference in population average iF between traffic and power plant emissions, while in this study the difference was 1.4-fold. Thus, the iFs evaluated by Tainio et al. (2009) tend to underestimate the emission-exposure relationships, especially for traffic and possibly for domestic combustion primary PM2.5 emissions, since emissions from these sources are released from low emission heights and in most cases, in relatively densely populated areas. This indicates that the emissionexposure variations between sources may be potentially even higher than those presented in this study, and that the emissionexposure variation could well have even greater impacts on the results of the integrated assessments.
The toxicity variation had major impacts on premature death estimates (Table 1). The exposure-response functions for different primary PM2.5 emission source categories were based on experts’ estimates of the most and least toxic substances in the PM2.5 mass (Cooke et al., 2007; Tuomisto et al., 2008). The substances that experts named as the least and the most toxic varied between the experts and therefore the combined estimate (that was used in this study) represents the maximum variation between the least and the most toxic substance in PM2.5 mass. Clearly, there are inherent uncertainties in using this kind of expert based approach, and the results for different emission source categories should be viewed more as indicative rather than quantitative.
Previous assessments have only rarely estimated toxicity differences between different particles, although several epidemiological studies have detected toxicity differences between PM from different sources (e.g. Laden et al., 2000; Lanki et al., 2006). In the European Externalities of Energy (ExternE) study, primary PM from traffic was evaluated to be 1.5 times more toxic than the average PM2.5 mass, while secondary sulfate and nitrate had a lower toxicity than the average PM2.5 mass (ExternE, 2005). The 2005 update of ExternE methodology did not include a sensitivity analysis and the impact of these toxicity variations in the assessment was not evaluated. A recent study on regional background exposure for PM2.5 in Europe used a 2.8 times higher RR estimate for primary PM2.5 mass in comparison to secondary PM2.5 mass (Andersson et al., 2009). They concluded that in Europe the exposure for secondary PM2.5 is higher than exposure for primary PM2.5 but due to toxicity differences, the magnitude of adverse health effects was similar.
This study was based on toxicity variation between different emission source categories. However, the toxicity is not dependent per se on the emission sources but rather on the chemical and physical properties of the inhaled PM. In an ideal case the exposureresponse functions would be based on the chemical and physical properties of inhaled PM. However, the current knowledge on the adverse health effects of PM2.5 is inadequate to permit this kind of exposure-response modeling.
Uncertainties
The uncertainties related to exposure-response functions were identified to possess the highest importance in the health effect estimates (Fig. 3). A similar result has been noted in our previous assessment studies for PM2.5 air pollution (Leino et al., 2008; Tainio et al., 2005, 2007) and in a number of other assessment studies (e.g. Künzli et al., 2000; Levy and Spengler, 2002).
The uncertainties related to input variables and for the model structure were evaluated with various methods. In a few cases, the uncertainty was based on previously published studies (e.g. emission uncertainties). For some of the input variables the uncertainty was defined with expert estimate (e.g. exposure-response functions). With respect to the other variables the uncertainty was estimated based on author judgment. Thus, different uncertainties were defined with different methods ranking from the expert study to the crude guess of the modeler. This raises a doubt of the comparativeness of different uncertainty estimates in the sensitivity analysis. However, the purpose of this study was more to demonstrate the model and to define those uncertainties that require a more formal analysis in future assessments than provide formal uncertainty assessment.
We assumed in this study much higher uncertainty to exposureresponse functions than previous assessment studies. For example, in the Pope et al., 2002 study, a Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle 1 \mu g m^{-3}} change in PM2.5 concentration was estimated to change non-accidental mortality between 0.15% and 1.00% (95% confidence interval). In this study, the corresponding variationwas 0.03e4.57% (for average exposure-response function). When testing the sensitivity of the model for different uncertainties by using similar uncertainty intervals as in Pope et al. (2002), the relative importance of exposure-response function uncertainties was still most important but only with small differences e.g. compared to the importance of the uncertainty in the dispersion model (data not presented). Thus, exposure-response function uncertainty remained important also while using less uncertain input variables.
We also assumed in this study that all the uncertainties are independent from each other. Thus, we did not assume any correlation between uncertainties. This could result in either under- or overestimation of total uncertainty of the model. However, we assume that this would not change significantly the results of this study since we compared mainly uncertainties that are uncorrelated (e.g. toxicity to iF).
Magnitude of health effects
The primary PM2.5 originating from the whole of Europe were estimated to cause in Finland approximately 566 premature deaths and to lower the average life expectancy by 0.12 years in 2000. The CAFE program has evaluated that PM2.5 were responsible for the premature deaths of 1270 Finns (Watkiss et al., 2005). When one takes into account that the CAFE study included both primary and secondary PM2.5, the results between CAFE and this study are not in disagreement. Over half of the adverse health effects in this study were due to long-range transported primary PM2.5 from other European countries. The exposure for local emission sources is probably underestimated in this study due to reasons discussed earlier.
We estimated that traffic-originated primary PM2.5 emissions from Finland caused approximately 85 premature deaths in Finland in 2000. In our previous studies, we have estimated that primary PM2.5 from local buses in Helsinki region would be responsible for 18 premature deaths in 2020 (Tainio et al., 2005) and that the primary PM2.5 due to the heavy-duty fleet (including buses) account for 34 premature deaths per year in the Helsinki metropolitan area (Leino et al., 2008). In our previous studies, the exposure was based on personal measurements of PM2.5 and the sources were identified with the source apportionment method. The comparison of these two studies therefore indicate that this study may underestimate the health effects for traffic-originated primary PM2.5, most probably due to underestimated exposure.
The magnitude of underestimation in exposure can also be estimated by comparing the measured PM concentrations to modeled ones. In this study the average population exposure in Finland for all primary PM2.5 emission sources included in the computations combined was less than 1Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu g m^{-3}} . For comparison, in 2001 the average PM2.5 concentrations, including secondary PM, in Helsinki region varied between 8Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu g m^{-3}} and 9Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu g m^{-3}} at two measurement stations (YTV, 2002). Although Helsinki is more polluted than the rest of the Finland, a substantial fraction of the measured PM2.5 are secondary PM, and present study did not include non-anthropogenic sources, less than 1Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu g m^{-3}} average exposure seems an underestimation of exposure.
The Finnish anthropogenic primary PM2.5 emissions were estimated in this study to be 30.8 (95% CI 27e36) Gg/a for the year 2000. In the European Thematic Strategy on Air Pollution assessment, in the year 2000 the primary PM2.5 emissions for Finland were 28 Gg/a based on the RAINS model (Amann et al., 2006). The higher emission strength in this study in comparison to RAINS is due to different emission source definitions of the traffic non-tailpipe emissions (RAINS do not include traffic induced dust suspension). Another emission inventory maintained by European Monitoring and Evaluation Programme (EMEP) and based on country submissions of national inventories estimated 38.2 Gg/ a primary PM2.5 emissions for Finland for 2000 (Vestreng et al., 2006). The higher value is mainly due to the outdated estimate of domestic wood combustion emissions in the EMEP database (Karvosenoja, 2008).
We assumed no threshold for the primary PM2.5 induced premature deaths. The previous assessments have shown that a threshold value can have major impact on the results of the assessment (e.g. Künzli et al., 2000). There have been several statistical attempts to define a threshold for PM2.5 air pollution. For example, Schwartz et al. (2002, 2008) have studied nonlinearities in exposure-response functions in order to define a threshold for shortterm( acute) and long-term (chronic) effect of PM2.5, respectively, but neither of these studies detected any threshold value for PM2.5. The World Health Organization working group stated in 2003 that epidemiological studies have been unable to identify any threshold for PM2.5 and that it is likely that the PM2.5 is harmful in the population since all populations contain susceptible individuals (WHO, 2003). However, in the expert elicitation study conducted by U.S. Environmental Protection Agency (EPA), a number of experts gave separate exposure-response coefficients and/or plausibility for the PM2.5 air pollution mortality impact of low exposure levels (usually below 10Failed to parse (SVG (MathML can be enabled via browser plugin): Invalid response ("Math extension cannot connect to Restbase.") from server "https://wikimedia.org/api/rest_v1/":): {\displaystyle \mu g m^{-3}} ) (Roman et al., 2008). Whether or not there is any threshold remains one of the important undefined uncertainties.
Conclusions
We have utilized an integrated assessment model to estimate the adverse health effects mainly due to anthropogenic primary fine particulate matter originating from different emission source categories. The variations in both emission-exposure and toxicities between source categories had significant impacts for the assessment results, especially for traffic-originated primary fine particulate matter. This kind of information is important for the rank ordering of the effectiveness of the available mitigation actions. The main uncertainties in the model were related to exposure-response functions and to the estimation of emission-exposure relationships for different source categories.
Acknowledgements
This study was done as a part of the projects KOPRA (funded by the Ministry of the Environment, Finland, Grant YM119/481/2002, the National Technology Agency of Finland (Tekes), Grant 616/31/ 02 and the Helsinki Metropolitan Area Council (YTV), Grant 135/ 03), BIOHER (funded by the Academy of Finland, Grant 10155) and PILTTI (funded by the Ministry of the Environment, Finland, Grant YM57/065/2005). The work relates to projects INTARESE (funded by European Union, Grant 018385-2), MEGAPOLI (funded by European Union) and SCUD (funded by the Academy of Finland, Grant 111775). Marko Tainio was supported by personal grant from the Graduate School in Environmental Health. We would like to thank Dr. Ewen MacDonald for checking the English language.
Appendix. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.atmosenv.2010.02.036 .
References
Amann, M., Asman, W., Bertok, I., Cofala, J., Heyes, J., Klimont, Z., Posch, M., Schöpp, W., Wagner, F., Hettelingh, J.-P., 2006. Emission control scenarios that meet the environmental objectives of the thematic strategy on air pollution. Laxenburg, Austria: NEC scenario analysis report Nr. 2. International Institute for Applied Systems Analysis (IIASA).
Andersson, C., Bergström, R., Johansson, C., 2009. Population exposure and mortality due to regional background PM in Europe long-term simulations of source-region and shipping contributions. Atmospheric Environment 43, 3614-3620.
Bennett, D.H., McKone, T.E., Evans, J.S., Nazaroff, W.W., Margni, M.D., Jolliet, O., Smith, K.R., 2002. Defining intake fraction. Environmental Science & Technology 36, 206a-211a.
Cooke, R.M., Wilson, A.M., Tuomisto, J.T., Morales, O., Tainio, M., Evans, J.S., 2007. A Probabilistic characterization of the relationship between fine particulate matter and mortality: elicitation of European experts. Environmental Science & Technology 41, 6598-6605.
ExternE - Externalities of Energy - Methodology 2005 Update. Luxembourg: Office for Official Publications of the European Communities. Available at: http://www.externe.info/brussels/methup05a.pdf.
Fann, N., Fulcher, C.M., Hubbell, B.J., 2009. The influence of location, source, and emission type in estimates of the human health benefits of reducing a ton of air pollution. Air Quality, Atmosphere, and Health 2, 169-176.
Hopke, P.K., Ito, K., Mar, T., Christensen, W.F., Eatough, D.J., Henry, R.C., Kim, E., Laden, F., Lall, R., Larson, T.V., Liu, H., Neas, L., Pinto, J., Stolzel, M., Suh, H., Paatero, P., Thurston, G.D., 2006. PM source apportionment and health effects: 1. Intercomparison of source apportionment results. Journal of Exposure Science & Environmental Epidemiology 16, 275-286.
Karvosenoja, N., 2008. Emission Scenario Model for Regional Air Pollution. Monographs of the Boreal Environment Research No. 32. Available at: http://www.ymparisto.fi/download.asp?contentid¼92446&lan¼en.
Karvosenoja, N., Tainio, M., Kupiainen, K., Tuomisto, J.T., Kukkonen, J., Johansson, M., 2008. Evaluation of the emissions and uncertainties of PM2.5 originated from vehicular traffic and domestic wood combustion in Finland. Boreal Environment Research 13, 465-474.
Künzli, N., Kaiser, R., Medina, S., Studnicka, M., Chanel, O., Filliger, P., Herry, M., Horak, F., Puybonnieux-Texier, V., Quenel, P., Schneider, J., Seethaler, R., Vergnaud, J.C., Sommer, H., 2000. Public-health impact of outdoor and trafficrelated air pollution: a European assessment. Lancet 356, 795-801.
Kupiainen, K.J., Karvosenoja, N., Porvari, P., Johansson, M., Tainio, M., Tuomisto, J.T., 2006. Emissions of primary carbonaceous particles, their uncertainties and spatial allocation in Finland. In proceedings from the IUAPPA regional conference/ 17th EFCA speciality conference, September 6-8, 2006, Lille, France.
Laden, F., Neas, L.M., Dockery, D.W., Schwartz, J., 2000. Association of fine particulate matter from different sources with daily mortality in six US cities. Environmental Health Perspectives 108, 941-947.
Lanki, T., de Hartog, J.J., Heinrich, J., Hoek, G., Janssen, N.A.H., Peters, A., Stolzel, M., Timonen, K.L., Vallius, M., Vanninen, E., Pekkanen, J., 2006. Can we identify sources of fine particles responsible for exercise-induced ischemia on days with elevated air pollution? The ULTRA study. Environmental Health Perspectives 114, 655-660.
Leino, O., Tainio, M., Tuomisto, J.T., 2008. Comparative risk analysis of dioxins in fish and fine particles from heavy-duty vehicles. Risk Analysis 28, 127-140.
Levy, J.I., Spengler, J.D., 2002. Modeling the benefits of power plant emission controls in Massachusetts. Journal of the Air & Waste Management Association 52, 5-18.
Pope, C.A., Burnett, R.T., Thun, M.J., Calle, E.E., Krewski, D., Ito, K., Thurston, G.D., 2002. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA - Journal of the American Medical Association 287, 1132-1141.
Pope, C.A., Dockery, D.W., 2006. Health effects of fine particulate air pollution: lines that connect. Journal of the Air & Waste Management Association 56, 709-742.
Roman, H.A., Walker, K.D., Walsh, T.L., Conner, L., Richmond, H.M., Hubbell, B.J., Kinney, P.L., 2008. Expert judgment assessment of the mortality impact of changes in ambient fine particulate matter in the US. Environmental Science & Technology 42, 2268-2274.
Schwartz, J., Laden, F., Zanobetti, A., 2002. The concentration-response relation between PM2.5 and daily deaths. Environmental Health Perspectives 110, 1025-1029.
Schwartz, J., Coull, B., Laden, F., Ryan, L., 2008. The effect of dose and timing of dose on the association between airborne particles and survival. Environmental Health Perspectives 116, 64-69.
Tainio, M., Tuomisto, J.T., Hänninen, O., Aarnio, P., Jantunen, M., Pekkanen, J., 2005. Health effects caused by primary fine particulate matter (PM2.5) emitted from busses in Helsinki metropolitan area, Finland. Risk Analysis 25, 151-160.
Tainio, M., Tuomisto, J.T., Hänninen, O., Ruuskanen, J., Jantunen, M.J., Pekkanen, J., 2007. Parameter and model uncertainty in a life-table model for fine particles (PM2.5): a statistical modeling study. Environmental Health: a Global Access Science Source 6.
Tainio, M., Sofiev, M., Hujo, M., Tuomisto, J.T., Loh, M., Jantunen, M.J., Karppinen, A., Kangas, L., Karvosenoja, N., Kupiainen, K., Porvari, P., Kukkonen, J., 2009. Evaluation of the intake fractions of primary fine particulate matter originated from various source categories. Atmospheric Environment 43, 1962-1971.
Tuomisto, J.T., Wilson, A., Evans, J.S., Tainio, M., 2008. Uncertainty in mortality response to airborne fine particulate matter: combining European air pollution experts. Reliability Engineering & System Safety 93, 732-744.
UN (United Nation), 2008. Population division of the department of economic and social affairs of the United Nations Secretariat, world population prospects: the 2006 revision and world urbanization prospects: the 2005 revision Available at: http://esa.un.org/unpp (accessed 06.02.03).
Vestreng, V., Rigler, E., Adams, M., Kindbom, K., Pacyna, J.M., Denier van der Gon, H., Reis, S., Travnikov, O., 2006. Inventory review 2006. Emission data reported to the LRTAP Convention and NEC Directive: stage 1, 2 and 3 review, and evaluation of inventories of HMs and POPs. MSC-W Technical report 1/2006. Available at: http://emep.int/publ/reports/2006/emep_technical_1_2006.pdf.
Watkiss, P., Pye, S., Holland, M., 2005. Baseline Scenarios for Service Contract for carrying out cost-benefit analysis of air quality related issues, in particular in the clean air for Europe (CAFE) programme. AEAT/ED51014/ Baseline Issue 5.
WHO (World Health Organization), 2003. Health aspects of air pollution with particulate matter, ozone and nitrogen dioxide. Report on a WHO working group. Bonn, Germany 13e15 January 2003. Available at: http://www.euro.who.int/document/e79097.pdf.
WHO (World Health Organization), 2007. Health Relevance of Particulate Matter from Various Sources. Report on a WHO Workshop. WHO, Bonn, Germany.
Wolff, S.K., 2000. Evaluation of Fine Particle Exposures, Health Risks and Control Options. Department of Environmental Health, Harvard School of Public Health, Boston, U.S.
YTV (Helsinki Metropolitan Area Council), 2002. Ilmanlaatu pääkaupunkiseudulla vuonna 2001. (Air quality in Helsinki metropolitan area in 2001). Pääkaupunkiseudun Julkaisusarja C 17 (2002).