Benefit-risk assessment of cinnamon
| Moderator:Anto.guzzon (see all) |
|
|
| Upload data
|
For a subsequent case study assessment by Plantlibra, see Benefit-risk assessment of cinnamon (password-protected).
15 July 2010
EMA/HMPC/246773/2009
Committee on Herbal Medicinal Products (HMPC)
Assessment report on Cinnamomum verum J. S. Presl (Cinnamomum zeylanicum Nees), cortex and corticis aetheroleum[1]
Draft
Based on Article 10a of Directive 2001/83/EC as amended (well-established use)
Based on Article 16d(1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use)
| Herbal substance(s) (binomial scientific name of the plant, including plant part) | Cinnamomum verum J. S. Presl. Nees, dried bark |
|---|---|
| Herbal preparation(s) | a) Comminuted herbal substance
b) Liquid extract (DER 1:1) extraction solvent: 70% ethanol c) Tincture (ratio of herbal substance to extraction solvent 1:5) extraction solvent 70% ethanol d) Essential oil obtained by steam distillation from the cortex |
| Pharmaceutical forms | Comminuted herbal substance as herbal tea for oral use.
Herbal preparation in liquid dosage forms. The pharmaceutical form should be described by the European Pharmacopoeia full standard term. |
Note: This draft Assessment Report is published to support the release for public consultation of the draft Community herbal monograph on Cinnamomum verum J. S. Presl, cortex and corticis aetheroleum. It should be noted that this document is a working document, not yet fully edited, and which shall be further developed after the release for consultation of the monograph. Interested parties are welcome to submit comments to the HMPC secretariat, which the Rapporteur and the MLWP will take into consideration but no ‘overview of comments received during the public consultation’ will be prepared in relation to the comments that will be received on this draft assessment report. The publication of this draft assessment report has been agreed to facilitate the understanding by Interested Parties of the assessment that has been carried out so far and led to the preparation of the draft monograph.
Contents
- 1 Introduction
- 2 Historical data on medicinal use
- 3 Non-Clinical Data
- 3.1 Overview of available pharmacological data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
- 3.2 Overview of available pharmacokinetic data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
- 3.3 Overview of available toxicological data regarding the herbal substance(s)/herbal preparation(s) and constituents thereof
- 4 Clinical data
- 5 Clinical Safety/Pharmacovigilance
- 5.1 Overview of toxicological/safety data from clinical trials in humans
- 5.2 Patient exposure
- 5.3 Adverse events and serious adverse events and deaths
- 5.4 Laboratory findings
- 5.5 Safety in special populations and situations
- 5.6 Intrinsic (including elderly and children) /extrinsic factors
- 5.7 Drug interactions
- 5.8 Use in pregnancy and lactation
- 5.9 Overdose
- 5.10 Drug abuse
- 5.11 Withdrawal and rebound
- 5.12 Effects on ability to drive or operate machinery or impairment of mental ability
- 5.13 Overall conclusions on clinical safety
- 6 Overall conclusions
Introduction
Description of the herbal substance(s), herbal preparation(s) or combinations thereof
- Herbal substance(s)
1. Cinnamomum verum J. S. Presl. (=Cinnamomum zeylanicum Nees), dried bark, freed from the outer cork and the underlying parenchyma (ESCOP, 2003; European Pharmacopoeia 6.2, 2009).
2. Cinnamomum verum J. S. Presl. is also known by the synonym Cinnamomum zeylanicum Blume and is member of Lauraceae family (Keller 1992).
This assessment report does not evaluate Cinnamomum aromaticum Nees (synonym: Cinnamomum cassia Blume), cortex although they are both comparable in composition and widely used in flavouring agents in foods and in pharmaceutical and cosmetic preparations (Kommission E, 1990; Barnes et al., 2007).
The drug consists of the dried bark, freed from the outer cork and the underlying parenchyma, of the shoots grown cut stock of Cinnamomum verum J. S. Presl. The matt pieces of bark, 0.2-0.7 mm thick and in the form of single or double compound quills, are light brown on the outside and somewhat darker on the inside. The surface is longitudinally striated and the fracture is short and splintery. It contains not less than 12 ml/kg essential oil obtained by steam distillation It has a characteristic and pleasantly aromatic odour. Its taste is pungently spicy, somewhat sweet and mucilaginous, and only slightly sharp (Bisset, 1994).
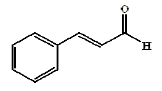
Cinnamon bark contains up to 4% of essential oil consisting primarily of cinnamaldehyde (60-75%), cinnamyl acetate (1-5%), eugenol (1-10%) (WHO Vol. 1999), β-caryophyllene(1-4%), linalool (1-3%) and 1.8-cineole (1-2%) (ESCOP, 2003).
Other constituents are oligopolymeric procyanidins, cinnamic acid, phenolic acids, pentacyclic diterpenes cinnzeylanol and it's acetyl derivative cinnzeylanine and the sugars mannitol, L-arabino-D-xylanose, L-arabinose, D-xylose, α-D-glucane as well as mucilage polysaccharides (Hänsel et al., 1992, ESCOP, 2003).
The essential oil of the bark is described in the European Pharmacopoeia 6.2 (2009) . There exists a summary report on the essential oil of cinnamon bark. This report has been made by the Committee for Veterinary Medicinal Products. According to this information the oil mainly contains cinnamaldehyde (55-76%), eugenol (5-18%) and saffrole (up to 2%). This document refers also to human use (CVMP 2000).
Cinnamon bark oil may be adulterated with cinnamon leaf oil and oil of cassia (Price & Price, 2007).
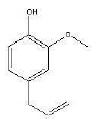
Figure 2: eugenol
- Herbal preparation(s)
a) Comminuted herbal substance
b) Liquid extract (DER 1:1) extraction solvent: 70% ethanol
c) Tincture (ratio of herbal substance to extraction solvent 1:5) extraction solvent: 70% ethanol
d) Essential oil obtained by steam distillation from the cortex
- Combinations of herbal substance(s) and/or herbal preparation(s) including a description of vitamin(s) and/or mineral(s) as ingredients of traditional combination herbal medicinal products assessed, where applicable.
See overview data from member states.
Vitamin(s)
The drug contains per 100 g:
- vitamin A: 260 IU
- thiamine: 0.02 mg
- riboflavin: 0.14 mg
- niacin: 1.3 mg
- ascorbic acid: 28 mg
(Duke, 1988)
Mineral(s)
The drug contains per 100 g:
- Ca: 1.228 mg
- P: 61 mg
- Fe: 38 mg
- Mg: 56 mg
- Na: 26 mg
- K: 500 mg
- Zn: 2 mg
(Duke, 1988)
Information about products on the market in the Member States
Regulatory status overview
| Member State | Regulatory Status | Comments (not mandatory field) | |||
|---|---|---|---|---|---|
| MA | TRAD | Other TRAD | Other specify: | ||
| Austria | Combined preparations authorized | ||||
| Belgium | x | Only in food supplement | |||
| Bulgaria | No authorized or registered preparations | ||||
| Cyprus | Not known | ||||
| Czech Republic | x | Combined preparations authorized | |||
| Denmark | x | Only combined preparations with cinnamon as a flavouring agent | |||
| Estonia | Combined preparations authorized | ||||
| Finland | Not known | ||||
| France | Not known | ||||
| Germany | x | x | Only herbal tea and combined products with cortex and essential oil | ||
| Greece | No authorized or registered preparations | ||||
| Hungary | Not known | ||||
| Iceland | No authorized or registered preparations | ||||
| Ireland | No authorized or registered preparations | ||||
| Italy | No authorized or registered preparations | ||||
| Latvia | Not known | ||||
| Liechtenstein | Not known | ||||
| Lithuania | x | Essential oil and combined preparations authorized | |||
| Luxemburg | Not known | ||||
| Malta | No authorized or registered preparations | ||||
| The Netherlands | No authorized or registered preparations | ||||
| Norway | No authorized or registered preparations | ||||
| Poland | x | Only combined preparations | |||
| Portugal | No authorized or registered preparations | ||||
| Romania | Not known | ||||
| Slovak Republic | No authorized or registered preparations | ||||
| Slovenia | No authorized or registered preparations | ||||
| Spain | x | Food supplements with essential oil (5 drops 3x/d) | |||
| Sweden | Not known | ||||
| United Kingdom | x | Only combined products | |||
MA: Marketing Authorisation
TRAD: Traditional Use Registration
Other TRAD: Other national Traditional systems of registration
This regulatory overview is not legally binding and does not necessarily reflect the legal status of the products in the MSs concerned.
Specific information about preparations in the EU
- Austria
Several liquid extracts of the drug in combination products for gastrointestinal complaints of as a flavouring excipient.
- Czech Republic
Solution for oral or external use containing extract from Melissae folium, Inulae radix, Angelicae radix, Zingiberis radix, Caryophylli flos, Galangae radix, Piperis nigri fructus, Gentianae radix, Myristicae semen, Aurantii pericarpium, Cinnamomi cortex, Cassiae flos, Cardamomi fructus.
- Germany
Cinnamon is one of the 13 ingredients of a solution for oral or external use (see above).
- Estonia
The same combination preparation as mentioned above.
- Lithuania
The same combination preparation as mentioned above and aetheroleum in a Polish product.
- Poland
Aromatol is a combined preparation containing Melissae aetheroleum, Caryophylli aetheroleum, Cinnamoni aetheroleum, Citri limoni aetheroleum, Menthae piperitae aetheroleum, Lavandulae aetheroleum and menthol in ethanol.
Search and assessment methodology
Historical data on medicinal use
Information on period of medicinal use in the Community
Cinnamon has been used as a spice for thousands of years; several references to it are found in the Bible. In Egypt, cinnamon was a spice used in embalming fluid. In Ayurvedic medicine, cinnamon bark was used as an antiemetic, antidiarrheal, antiflatulent, and general stimulant. The Portuguese found cinnamon trees growing in Sri Lanka (Ceylon) during the early 16th century; they subsequently imported cinnamon to Europe during the 16th and 17th centuries. The Dutch occupied Sri Lanka in the mid-17th century until the British captured the island in 1796. The East India Company then became the main exporter of cinnamon to Europe. The Dutch cultivated cinnamon in Java, and the exports of Ceylon cinnamon decreased as a result of heavy export duties. Nevertheless, Sri Lanka is the only regular supplier of cinnamon bark and leaf oils. The food industry prefers Ceylon cinnamon, but pharmaceutical manufacturers use both oils from Ceylon cinnamon (cinnamon oil) and from Chinese cinnamon (cassia oil) interchangeably. China is the main exporter of cassia cinnamon (Barceloux 2009).
Information on traditional/current indications and specified substances/preparations
The herbal preparations are in traditional medicinal use for over 30 years (Wahrig & Richter, 2009).
- The following indications have been reported for cinnamon:
Dyspeptic complaints such as gastrointestinal spasms, bloating and flatulence, loss of appetite and diarrhoea (ESCOP, 2003).
Cinnamon is used primarily as a taste enhancer and as a spice, and to some extent also in preparation of liquors.
Indications in folk medicine: diarrhoea, dyspeptic complaints, cold and flu, topical use for wound-cleaning. Except for dyspeptic complaints, the indications in folk medicine have not been sufficiently documented (Hänsel et al., 1992). The essential oil is used drop-wise (“cinnamondrops”) as a remedy on dysmenorrhoea and to stop bleeding (Bisset, 1994).
Another source lists the following indications: application as an astringent, germicide, and antispasmodic. Cinnamon was one of the early treatments for chronic bronchitis, treatment of impotence, frigidity, dyspnea, inflammation of the eye, leukorrhea, vaginitis, rheumatism, and neuralgia, as well as wounds and toothaches (Barceloux, 2009).
- Indications for combination preparations:
Czech Republic, Estonia and Lithuania: Solution for oral use as an adjuvant at mild psychovegetative disturbances such as loss of appetite and gastrointestinal complaints, mood changes and headache of different origin, nervosity, restlessness, sleep disorders and as an adjuvant in common cold. Externally used as mild remedy in neuritis, muscle pains, lumbago and gingivitis.
Specified strength/posology/route of administration/duration of use for relevant preparations and indications
Posology and method of administration:
Adolescents, adults:
dried bark 1.5-4 g daily: 0.5-1.0 g as an infusion three times daily (Barceloux, 2009; Hänsel et al., 1992; Delfosse 1998; ESCOP 2003; WHO 1999; Kommission E 1990; Barnes et al., 2007).
fluid extract 0.5-1.0 ml (1:1) extraction solvent: 70% ethanol V/V three times daily (Hänsel et al., 1992; ESCOP 2003; Barnes et al., 2007).
tincture 2-4 ml daily (1:5) extraction solvent: 70% ethanol V/V: to be given in 3 doses (Hänsel et al., 1992; ESCOP 2003; Barnes et al., 2007; Todd 1967).
aetheroleum 50-200 mg daily: to be given in 3 doses (CVMP 2000; Delfosse 1998; WHO 1999; Todd 1967).
spiritus (1 part of oil in 10 of ethanol 90%) 0.3 to 1.2 ml daily: to be given in 3 doses (Todd 1967).
All preparations are orally administered.
Elderly: the same dose as for adults
Method of administration
Oral use
If the symptoms persist during the use of the medicinal product, a doctor or a qualified health care practitioner should be consulted.
(Barnes et al., 2007; ESCOP, 2003; Kommission E, 1990)
Non-Clinical Data
Overview of available pharmacological data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
In vitro tests
Flatulence:
A foam generator as a model of flatulence for generating and assessing foams in digestive fluids in vitro. The effects of volatile oils, including cinnamon oil on gastric and intestinal foams were examined. Reductions in foam volume were observed in every case, although the effects were not as high as those produced by a combination of dimethicone and silica. m-Cresol, p-hydroxybenzaldehyde, isobutanol, menthol and phenoxyethanol also reduced foam volume, but anti-foaming activity could not be related to published data on the ability to produce a 50% inhibition of a standard response in guinea pig ileum to carbachol. It is suggested that carminative action is a combination of effects, one of which is a reduction of gastrointestinal foam (Harries et al., 1978).
Papaverine-like spasmolytic effects of cinnamon bark oil (EC50 41 mg/litre) and cinnamaldehyde were observed in tests on guinea pig tracheal and ileal smooth muscle.
The results were compared with the relaxant effects of catecholamines and phosphodiesterase inhibitors. In regard to the relaxant effects, the examined oils, including cinnamon bark oil, were more potent on the ileal than on the tracheal muscle. However, a small group of oils had a higher relaxant effect on the tracheal than on the ileal muscle. This was also found to be the case with eugenol, eugenol acetate and cinnamic aldehyde (components of cinnamon oil ) as well as with isoprenaline and phosphodiesterase inhibitors (Reiter et al., 1985).
Antifungal activities:
The antifungal activities are mainly contributed to the essential oil (in concentrations ranging from 1% to 0.0025 %) and its active compound cinnamaldehyde inhibiting the growth of fungi and yeasts as well as their mycotoxin production: e.g. Aspergillus clavus, A. niger, A. parasiticus, Candida albicans (Kalemba & Kunicka, 2003). Cinnamon bark oil also inhibits the growth of several dermatophytes. The inhibitory zone induced by cinnamon bark oil in solid media was 28 mm in diameter, comparable to the 20-25 mm zone induces by ketoconazole at 100 μg/ml (Lima et al., 1993). The essential oils most active constituents upon mycelial growth were also the most active against aflatoxinogenesis. However, aflatoxin synthesis was inhibited by the examined essential oils at higher extent than the mycelial growth (Tantaoui-Elaraki & Beraoud, 1994).
Antibacterial properties:
The essential oil was screened against four gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris) and two gram-positive bacteria (Bacillus subtilis and Staphylococcus aureus) at four different concentrations (1:1, 1:5, 1:10 and 1:20) using the disc diffusion method. The MIC of the active essential oils were tested using two fold agar dilution method at concentrations ranging from 0.2 to 25.6 mg/ml. Cinnamon oil exhibited a promising inhibitory effect, showing inhibitory activity, even at low concentration, on both gram-positive and gram-negative bacteria. The zone of inhibition above 7 mm in diameter was taken as positive result.
In general cinnamon oil showed inhibitory effect against P. aeruginosa (33.3 mm), B. subtilis (29.9 mm), P. vulgaris (29.4 mm), K. pneumoniae (27.5 mm) and S. aureus (20.8 mm) (Prabuseenivasan et al., 2006).
The antimicrobial activity against the food-borne pathogenic gram positive bacteria of Listeria monocytogenes Scott A, were studied in semi-skimmed milk incubated at 7°C for 14 days and at 35°C for 24 h. The MIC for cinnamon bark essential oil was 500 ppm. The effective concentration increased to 1,000 ppm when the semi-skimmed milk was incubated at 35°C for 24 h and the Minimum Bactericidal Concentration (MBC) was rated 3,000 ppm. The influence of the fat content of milk on the antimicrobial activity of the essential oil was tested in whole and skimmed milk. In milk samples with higher fat content, the antimicrobial activity of the essential oil was reduced. These results indicate the possibility of using essential oil of cinnamon in milk beverages as a natural antimicrobial (Cava et al., 2007).
Anti-inflammatory activity:
Various essential oils, including cinnamon bark oil, used in the treatment of rheumatism and inflammation as well as some of their main constituents and phenolic compounds known for their irritant and pungent properties were screened for activity as inhibitors of prostaglandin biosynthesis. A combination of a prostaglandin-synthesizing cyclo-oxygenase system from sheep seminal vesicles and an HPLC separation technique for the metabolites of arachidonic acid was used as test system. Cinnamon bark oil showed inhibitory cyclo-oxygenase activity. The active compound is probably eugenol (Wagner et al., 1986).
Effects on human spermatozoa:
The effect of cinnamon oil on human spermatozoa in vitro was studied. Fresh ejaculates were obtained from male partners of infertile couples. Cinnamon bark oil showed a spermicidal effect with a minimum concentration of 1:400 V/V. The percentage change in motility over control was calculated. The spermicidal action was confirmed by a supra-vital staining. Higher dilution of the volatile oil also was spermicidal when incubated with semen samples for a longer period (Buch et al., 1988).
Cytotoxicity:
Petroleum ether (25:1) and chloroform (68:1) extracts of cinnamon exhibited cytotoxic effects with respective ED50 values of 60 and 58 μg/ml in human cancer (KB) cells and 24 and 20 μg/ml in mouse leukemia L1210 cells (Chulasiri MU, 1984).
Cinnamaldehyde, also cytotoxic to rat hepatocytes, is the reactive cinnamyl species, since this compound is the most potent intracellular GSH depletor and exhibits the highest cytotoxicity in hepatocyte suspensions with a threshold cytotoxic concentration of 10-3 M. Predepletion of GSH with diethyl maleate and/or inhibition of -glutamylcysteine synthetase by l-buthionine S,R-sulfoximine reduces the threshold concentration for cytotoxicity. Intracellular glutathione was progressively depleted by cinnamaldehyde in a concentration-dependent manner from 10-4 to 10-3 M (Swales & Caldwell, 1992).
Antitproliferative activity
Trans-cinnamaldehyde (CA) and its analogues 2-hydroxycinnamaldehyde and 2 - benzoyloxycinnamaldehyde have been reported to possess antitumor activity. CA is also a known Nrf2 activator. Cinnamaldehyde analogues were synthesized and screened for antiproliferative and thioredoxin reductase (TrxR)-inhibitory activities. Whereas CA was weakly cytotoxic and TrxR inhibiting, hydroxy and benzoyloxy substitutions resulted in analogs with enhanced antiproliferative activity paralleling increased potency in TrxR inactivation. TrxR inactivation contributes at least partly to cinnamaldehye cytotoxicity. These Michael acceptor molecules can potentially be exploited for use in different concentrations in chemotherapeutic and chemo-preventive strategies (Chew et al., 2009).
In vivo tests
Spasmolytic and cardiovascular activity:
Effects of cinnamaldehyde on the cardiovascular and digestive systems were examined. A papaverine-like muscolotropic activity of cinnamaldehyde seemed to be involved in the vasodilatation. Cinnamaldehyde, administered intravenously at 5 and 10 mg/kg body weight, moderately inhibited both the rat stomach movement and the mouse intestinal propulsion and had a stimulating effect on the cardiovascular system. Concentrations of 10-4–10-5 g/ml were used in organ preparations. Gastric erosions produced in stressed mice were protected by oral administration of cinnamaldehyde. When administered intravenously to dogs at 5 and 10 mg/kg body weight, cinnamaldehyde had a dose-dependent hypotensive effect (Harada & Jano, 1975).
Other studies have shown that cinnamaldehyde is an inhibitor of stomach peristalsis in anaesthetised rats (5-20 mg/kg intravenously) and of the peristalsis in the gut of mice (250 mg/kg intraperitoneally). Cinnamaldehyde also stimulates bile secretion in rats (500 mg/kg), has central nervous system stimulating activity in rabbits (10-20 mg/kg intra-arterially) and inhibits motor activity in mice (250-1000 mg/kg oral doses) (CVMP, 2000).
Anti-nocinceptive activity:
Dry ethanolic extract of Cinnamomum zeylanicum administered orally to mice at 200 and 400 mg/kg body weight exhibited analgesic effects on the hot plate thermal stimulation and the acetic acid induced writhing tests on a dose dependent manner (Atta AH, 1998).
Anti-inflammatory activity:
Dry ethanolic extract of Cinnamomum zeylanicum administered orally to rats at 400 mg/kg body weight showed an anti-inflammatory effect only against chronic inflammation induced by cotton pellet granuloma indicating anti-proliferative effect (Atta AH, 1998).
Overall conclusions on pharmacology
The in vitro and in vivo tests on the spasmolytic activity of cinnamon bark oil and cinnamaldehyde show clear relaxant effect on the tracheal and ileal smooth muscles. The musculo-tropic activity of cinnamaldehyde also participates in vasodilatation. Anti-foaming activity may contribute to the carminative effect.
The essential oil has antimicrobial activity. It can inhibit the growth of fungi and yeasts like Aspergillus and Candida, including their mycotoxin production. Also the inhibition of dermatophytes has been observed. Inhibitory activity against aflatoxinogenesis is more pronounced than the inhibition of mycelial growth. Cinnamon oil also exhibited inhibitory activity towards both gram-positive and gram-negative bacteria in vitro. As for the cytotoxic effects, studies have been performed on mouse leukemia cells, rat hepatocytes and human spermatozoa and all studies have proven a certain degree of cytotoxicity.
The in vivo experiments on anti-inflammatory and anti-nociceptive activity of the ethanolic extracts of cinnamon showed positive results.
In general the pharmacological effects and the antimicrobial activity described are obtained with high concentrations of cinnamon oil. They partially support the traditional indication but they cannot support a well established use.
Overview of available pharmacokinetic data regarding the herbal substance(s), herbal preparation(s) and relevant constituents thereof
Metabolism
After application of cinnamaldehyde to rats benzoic acid and cinnamic acid were found in urine samples (Hänsel R et al., 1992).
The pharmacokinetics of cinnamic acid (CA) after oral administration of a decoction of Ramulus Cinnamomi (RC) 7.4 g/kg [containing CA 7.62×10-5 mol/kg and cinnamaldehyde (CNMA) 1.77×10-5 mol/kg], was compared with that after oral administration of pure CA 7.62×10-5 mol/kg in rats. Plasma concentrations of CA and hippuric acid (HA) were determined by HPLC. Pharmacokinetic parameters were calculated from the plasma concentration-time data. CA was quickly absorbed and then metabolized mainly into HA. The AUC (0-t) and AUC (0-∞) of CA were higher in RC group than those in pure CA group and the bioavailability of CA from RC was higher than that from pure CA. After intragastric administration of 3.79×10-4 mol/kg, CNMA was at least partially metabolized into CA in stomach and small intestine and almost completely metabolized into CA in liver before it is absorbed into blood in rats. The results showed that plasma CA in RC group might partly come from transformation of CNMA in RC (Chen et al., 2009).
Overall conclusions on pharmacokinetics
In vivo data on pharmacokinetics are available on cinnamic acid in cinnamon bark decoction as compared to pure cinnamic acid (Chen et al., 2009). Oral bioavailability of cinnamic acid was higher when administered as the decoction. Cinnamaldehyde seems to be highly susceptible to oxidation. Pharmacokinetic data on the essential oil are absent.
Overview of available toxicological data regarding the herbal substance(s)/herbal preparation(s) and constituents thereof
Acute toxicity:
The oral LD50 of cinnamon bark oil in rats has been determined as 4.16 g/kg and 3.4 ml/kg body weight. For cinnamaldehyde the oral LD 50 in rats is 2.22 g/kg bodyweight. The dermal LD50 of cinnamon bark oil in rabbits has been reported as 0.69 ml/kg and 0.59 mg/kg for cinnamaldehyde. The undiluted oil was severely irritating to the intact skin of rabbits and mildly irritating when applied to the backs of hairless mice; however, when tested at 8% in petrolatum it produced no irritation in 25 human subjects (ESCOP, 2003).
Sub-acute and chronic toxicity:
The acceptable daily intake of cinnamaldehyde was set to 700 μg/kg of bodyweight was set by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) but was not extended because of inadequate toxicity data. The ADI of eugenol is up to 2.5 mg/kg (CVMP, 2000). Cinnamaldehyde added to the diet of sprague dawley rats for 16 weeks at 10 g/kg resulted in slight swelling of hepatic cells and slight hyperkeratosis of the squamous portion of the stomach; the no-effect level was at 1000 and 2500 mg/kg (ESCOP, 2003).
In 90-day studies on mice given an oral dose of 100 mg/day/kg of an ethanolic extract C. zeylanici cortex (C. verum, crude drug/extract ratio not given) report inducing an increase in reproductive organ weights, sperm motility, sperm count. The study failed to illicit any spermatotoxic effect reported in earlier in vitro studies (CVMP, 2000).
Mutagenicity and genotoxitcity:
Cinnamon extracts, cinnamon bark oil and cinnamaldehyde showed no mutagenic potential in several studies using the Ames test. The Ames Salmonella reversion assay showed negative for cinnamaldehyde (Sekizawa & Shibamoto, 1982). Trans-cinnamaldehyde and trans-cinnamic acid were not mutagenic in five strains of Salmonella typhimurium. The mutagenicity tests were performed both without and with activation by rat and hamster liver microsomal preparations (Lijinsky & Andrews, 1980).
On the other hand, more studies, also using the Ames test, have shown mutagenic activity of cinnamon compounds. Cinnamon bark oil and cinnamaldehyde gave positive results in chromosomal aberration tests using Chinese hamster cell cultures as substrate (Ishidate et al., 1984). Positive results in the Ames test were also obtained for alcoholic extracts of cinnamon (Keller 1992).
Cinnamaldehyde and cinnamyl alcohol were positive in the Bacillus subtilis DNA-repair test. Both compounds were negative in the Escherichia coli WP2 uvrA reversion test (Sekizawa & Shibamoto, 1982; Keller 1992).
In the Bacillis subtilis DNA repair test (repair-recombinant proficient strains), using Bacillus subtilis strains H17 (rec+) and M45 (rec-), the petroleum ether and the chloroform extracts of C. verum showed mutagenicity. On the contrary, the ethanol extract showed no mutagenic activity (Ungsurungsie et al., 1984; Ungsurungsie et al., 1982).
Cinnamon oil and cinnamaldehyde gave positive results in the chromosomal aberration tests using Chinese hamster cell cultures (Keller 1992). Cinnamaldehyde showed genotoxic effects in Drosophila test systems. In another study an aqueous extract of cinnamon gave a negative result in a similar Drosophila test system (ESCOP, 2003; Keller 1992).
The results of the in vitro bacterial mutagenicity tests must be interpreted with care because the concentrations used were within the dose range where antimicrobial effects of cinnamaldehyde and cinnamon oil have been proven. Furthermore growth retardation due to antimicrobial effect is the reason for the reported ‘antimutagenic’ effect of cinnamaldehyde (Keller 1992).
Concentrations of 50, 100, 200, 300, 500, 1000 and 2000 μ/ml of the essential oil of Cinnamomum verum (Cinnamon) were tested in Salmonella typhymurium strains TA100 with and without rat liver S9 using Ames Salmonella reversion assay. Results: Without S9 fraction, increase in mutant colonies per plate was not observed in all used concentrations. Also with the S9 fraction none of the samples caused a significant increase in mutant colonies per plate (Shoeibi et al., 2009).
Carcinogenicity:
According to Keller (1992) unspecific cytotoxic effects in vitro are strongly influenced by the culture medium and other experimental conditions. On the other hand cinnamaldehyde inhibits thioredoxin reductase and can be considered as a candidate for cancer therapy and chemoprevention (see earlier Chew et al., 2009).
Teratogenicity:
Cinnamaldehyde was reported to exhibit teratogenic effects in chick embryos. The teratogenic dose was closely related to the toxic dose of cinnamaldehyde (0.5 mmol/embryo) with 58.2% malformations and 49 % lethality. However, a methanol extract of cinnamon given by gastric intubation was not teratogenic in rats (ESCOP, 2003).
Overall conclusions on toxicology
Cinnamon oil contains cinnamaldehyde which is an irritating and sensitizing component that could be the cause of dermatitis. Most of the available data are related to cinnamaldehyde. When using the essential oil or extracts of cinnamon the results are mostly negative. This makes data on mutagenicity of cinnamon rather contradictory and further investigation as to the beneficial role of the natural matrix is required. Investigations on mutagenicity and genotoxicity are insufficient to fully evaluate the carcinogenic risk of cinnamon. The data on teratogenicity are considered to be of limited value in risk assessment for humans (WHO Monographs, 1999).
In summary the data on toxicity are insufficient. Therefore a list entry cannot be recommended.
Clinical data
Clinical Pharmacology
Overview of pharmacodynamic data regarding the herbal substance(s)/preparation(s) including data on relevant constituents
A small scale clinical study by Khan et al., (2003) included 60 patients with type 2 diabetes, randomly divided into groups, were given capsules containing Cinnamomum cassia 1.3 or 6 g daily (part of the plant not specified). The capsules were given as an add-on therapy to antidiabetic medication. The cinnamon was consumed 40 days followed by a 20-day washout period. Effects on the blood glucose, triglyceride, total cholesterol, HDL cholesterol, and LDL cholesterol levels were measured. After 40 days, all three levels of cinnamon reduced the mean fasting serum glucose (18-29%), triglyceride (23-30%), LDL cholesterol (7-27%), and total cholesterol (12-26%) levels while no significant changes were noted in the placebo groups. Changes in HDL cholesterol were not significant.
An aqueous purified extract of Cinnamomum cassia (plant part not specified) was administered to 79 diabetes mellitus type 2 patients three times a day for 4 months. The amount of aqueous cinnamon extract corresponded to 3 g of cinnamon powder per day. There was a higher reduction of fasting plasma glucose level (10.3%) in the cinnamon group than in the placebo group (3.4%). No significant differences were observed regarding HbA1c, lipid profiles or differences between the pre- and post-intervention levels of these variables. The decrease in plasma glucose correlated significantly with the baseline concentrations, indicating that subjects with a higher initial plasma glucose level may benefit more from cinnamon intake (Mang et al., 2006).
The influence of Cinnamomu cassia on glucose homeostasis was investigated in 7 lean healthy volunteers (26 + 1 years; BMI 24.5 + 0.3). They underwent an oral glucose tolerance test (OGTT) supplemented with either placebo or 5 g powdered Cinnamomum cassia (most probably bark powder). Cinnamon ingestion reduced total plasma glucose response (AUC) to 75 g glucose orally ingested. When ingested with the glucose, cinnamon reduced the plasma response to glucose with 13%. When ingested 12 hours prior to glucose, this reduction was 10% (both p<0.05). This study proves an immediate and long lasting (12 hours) effect of Cinnamom cassia on glucose in plasma (Solomon and Blannin, 2007).
Eight male volunteers (aged 25 ± 1 years, body mass 76.5 ± 3.0 kg, BMI 24.0 ± 0.7 kg/m²; mean ± SEM) underwent two 14-day interventions involving cinnamon (6 capsules each containing 500 mg Cinnamomum cassia, part of the plant not specified) or placebo supplementation. Placebo supplementation was continued for 5 days following this 14 day period. Oral glucose tolerance tests (OGTT) were performed on days 0, 1, 14, 16, 18, and 20. Cinnamon ingestion reduced the glucose response to OGTT on day 1 (-13.1 ± 6.3% vs. day 0; P = 0.05) and day 14 (-5.5 ± 8.1% vs. day 0; P = 0.09). Cinnamon ingestion also reduced insulin responses to OGTT on day 14 (-27.1 ± 6.2% vs. day 0; P = 0.05), as well as improving insulin sensitivity on day 14 (vs. day 0; P = 0.05). These effects were lost following cessation of cinnamon feeding. Cinnamomum cassia may improve glycaemic control and insulin sensitivity, but the effects are quickly reversed (Solomon and Blannin, 2009).
A limited number of subjects (N = 22) with impaired fasting blood glucose and a BMI ranging from 25 to 45, were enrolled in a double-blind placebo-controlled trial., Subjects were given capsules containing either a placebo or 250 mg of a dried aqueous extract of Cinnamomum cassia (Cinnulin PF, part of the plant not specified) two times per day for 12 weeks.
Plasma malondialdehyde (MDA) concentrations were assessed using high performance liquid chromatography and plasma antioxidant status was evaluated using ferric reducing antioxidant power (FRAP) assay. Erythrocyte Cu-Zn superoxide (Cu-Zn SOD) activity was measured after hemoglobin precipitation by monitoring the auto-oxidation of pyrogallol and erythrocyte glutathione peroxidase (GPx) activity by established methods.
FRAP and plasma thiol (SH) groups increased, while plasma MDA levels decreased in subjects receiving the cinnamon extract. Effects were more pronounced after 12 than 6 weeks. There was also a positive correlation (r = 0.74; p = 0.014) between MDA and plasma glucose. This study supports the hypothesis that the inclusion of water soluble compounds of Cinnamomum cassia reduces risk factors associated with diabetes and cardiovascular disease (Roussel et al., 2009).
Rudowska (2009) made an overview of studies done with Cinnamomum cassia to influence blood glucose. They consider the herbal preparation as a functional food. According to this author fasting blood glucose seems to be reduced by cinnamon supplements in men and women with the metabolic syndrome. Also a high dose (6 g/d) of cinnamon with rice pudding reduces postprandial blood glucose and delays gastric emptying without affecting satiety.
However some studies failed to replicate these findings. Cinnamon supplementation (1.5 g/d) did not improve whole-body insulin sensitivity, oral glucose tolerance or blood lipid profile in postmenopausal patients with type 2 diabetes. Prospective randomized controlled trials indicate that the use of cinnamon does not confirm to improve Fasting Blood Glucose (FBG), glucosylated heamoglobin (HbA1c) or lipid parameters in patients with type 1 or type 2 diabetes.
Overall conclusions on pharmacodynamics
Clinical pharmacological data of Cinnamomum verum preparations do not exist. The plausibility of the efficacy is based on the traditional medicinal use and supported by the non clinical data.
Recent Studies with a different Cinnamomum species, C. cassia, showed a hypoglycemic effect (Khan, 2003; Mang, 2006). The studies are not related to C. verum.
General data on the base of other plant species are not considered in the labelling. From the traditional use of C. verum no clinical hypoglycaemic effects are reported.
Overview of pharmacokinetic data regarding the herbal substance(s)/preparation(s) including data on relevant constituents
No pharmacokinetic data are available.
Overall conclusions on pharmacokinetics
Clinical data on absorption, distribution and pharmacokinetic interactions are not available.
Clinical Efficacy
Dose response studies
Not available.
Clinical studies (case studies and clinical trials)
Only available for Cinnamomum cassia (see 4.1.1).
Clinical studies in special populations (e.g. elderly and children)
Not available.
Overall conclusions on clinical pharmacology and efficacy
Currently there’s no data available on clinical efficacy.
Clinical Safety/Pharmacovigilance
Overview of toxicological/safety data from clinical trials in humans
Patient exposure
Adverse events and serious adverse events and deaths
Cinnamon bark oil
Undiluted cinnamon bark oil was mildly irritating when applied to the backs of hairless mice and strongly irritating under occlusion to both intact and abraded rabbit skin.
The preparation of 8% in petrolatum did not cause irritation but gave sensitization reactions in humans. Also cases of contact sensitivity to a dentifrice containing the oil have been reported.
It is recommendable to avoid the use of the oil per os in liver conditions, alcoholism and when taking paracetamol because of glutathione depleting action of cinnamaldehyde (Price & Price, 2007).
Cinnamaldehyde, one of the components of the essential oil, 5% in petrolatum is a skin irritant. Cinnamon oil caused second-degree burns of after 48 hours of contact on the skin of an 11-year-old boy (Keller, 1992).
Extensive reporting on side effects of cinnamon is done by Barceloux (2009). Most case reports of toxicity from cinnamon oil involve local irritation and allergic reactions to cinnamon oil as a constituent of personal hygiene (toilet soaps, mouthwash, toothpaste, perfumes, mud baths), beverages (colas, vermouth, bitters), or baking products. Allergic reactions include contact dermatitis, perioral dermatitis, cheilitis, stomatitis, gingivitis, glossitis, chronic lichenoid mucositis, contact urticaria, 24 and rarely immediate hypersensitivity reactions (asthma, urticaria). Clinical manifestations of intraoral reactions include pain, swelling, erythema, ulcerations, fissures, vesicles, and white patches. These reactions are local, and distal skin involvement is rare. Occupational allergic contact dermatitis from spices is rare, and typically involves the hands. More common food sensitizers include carrot, cucumber, tomato, melon, fish, potato, orange, green pepper, onion, red cherry, and garlic. Cinnamon oil contains local mucous membrane irritants such as cinnamaldehyde and cinnamic acid.
Prolonged skin contact (48 hours) from a cinnamon oil spill produced superficial partial-thickness burns (Ref. xxx) Chronic use of cinnamon flavored gum can produce sub-mucosal inflammation and alteration of the surface epidermis resembling oral leukoplakia, manifest on biopsy by acanthosis, hyperkeratosis, parakeratosis, plasma cell infiltration, fibrosis of the lamina, and focal atypia. These changes are not pathognomonic for cinnamon-induced mucositis. The differential diagnosis of chronic mucositis associated with hypersensitivity to cinnamon includes local trauma, smokeless tobacco keratosis (snuff dipper’s lesion), hyperkeratosis, lichen planus, lupus erythematosus, candidiasis, premalignant lesions, lichenoid mucositis, and carcinoma.
The severity of the local mucosal reaction depends on the duration of cinnamon-gum chewing. In contrast to the diffuse gingival reaction associated with cinnamon-flavored toothpaste, oral lesions associated with gum chewing occur on the lateral border of the free tongue or adjacent buccal mucosa. Sequelae of contact dermatitis associated with cinnamon include desquamation and hyperpigmentation. Although a case report associated the development of squamous cell carcinoma of the tongue with prolonged use of cinnamon gum, the International Agency for Research on Cancer (IARC) and the US National Toxicology Program do not list cinnamon as a potential carcinogen. School-aged children abuse cinnamon oil by sucking on toothpicks or fingers dipped in the oil. Reported effects include facial flushing, sensation of warmth, and intraoral hyperesthesias. Although nausea and abdominal pain may occur, systemic symptoms do not usually result from this type of exposure.
Ingestion of cinnamon oil may cause central nervous system depression, predisposing the patient to aspiration pneumonia.
Serious adverse events and deaths
Severe reactions such as anaphylaxis have not been reported. All cases with side effects recovered when the cinnamon containing preparation was withdrawn. Nevertheless preparations with more than 0.01% cinnamaldehyde are suspect according to Keller (1992).
Laboratory findings
No data available.
Safety in special populations and situations
Patients with an allergy to cinnamon, cinnamaldehyde or Peru balsam (WHO monographs, 1999). Cinnamon bark oil must be regarded as a potential sensitizer on the skin. It should not be used on young children and old people. Because of the experimentally demonstrated glutathione depleting activity of cinnamaldehyde, it is recommendable to avoid administering cinnamon bark oil per os when patients suffer from liver conditions, in case of alcoholism and when taking paracetamol (Price & Price, 2007).
Intrinsic (including elderly and children) /extrinsic factors
No special studies about the use in children or elderly exist.
Drug interactions
No drug interactions are mentioned for Cinnamomum zeylanicum.
Cinnamomum cassia bark (2 g in 100 ml) markedly retarded the in vitro dissolution of tetracycline. HCl from gelatine capsules: only 20% dissolved within 30 minutes in contrast to 97% when only water was used. The effect was attributed to the adsorption of the antibiotic on the particles. There are no further investigations to establish which individual constituents of cassia bark (volatile oil, cinnamaldehyde or mucilage) are responsible for this effect. As a dose of 1 to 2 g cinnamon is not uncommon, care should be taken not to use tetracyclines together with cinnamon at that dose level (Keller, 1992).
Use in pregnancy and lactation
Only limited data available. The data are not sufficient for an adequate benefit/risk assessment. In accordance with general medicinal practice, Cinnamomi cortex products should not be used during pregnancy and lactation (WHO monographs, 1999).
Cinnamon was used in antiquity and the middle ages as abortifacient. It must be taken into account that in old sources cinnamon (cassia) was confused with Cassia fistulosa which contains anthraquinone (Keller, 1992).
An unspecified ‘large amount of cinnamon’ was reported to have caused methemoglobinemia, hematuria, albuminuria and cylindruria in pregnant women. Abortion was not reported. The daily intake of 100 drops of a non specified tincture of cinnamon did not result in abortion (Keller, 1992).
Overdose
Cinnamon bark oil and cinnamaldehyde in doses over 0.2 g/day (equivalent to 15-20 g of crude drug) have irritant properties (ESCOP, 2003).
A case report of massive ingestion of 60 ml cinnammon oil has been published. The patient, a 7.5-year-old child, immediately felt a burning sensation in the throat and stomach, experienced a double vision and had a warm dry skin. The child exhibited sleepiness, dizziness and a rapid pulse (100 beats/min.) (Pilapil, 1989).
Drug abuse
Cases of cinnamon oil abuse were documented in adolescents and children. Sucking on toothpicks or fingers which had been dipped in cinnamon oil was the primary method of abuse. The subjects experienced a rush or sensation of warmth, facial flushing, and oral burning. Some children complained of nausea or abdominal pain but no systemic effects were reported (Perry et al., 1990).
Withdrawal and rebound
None known
Effects on ability to drive or operate machinery or impairment of mental ability
None known.
Overall conclusions on clinical safety
The efficacy of Cinnamomum verum is plausible on the basis of long standing use and experience. The traditional use over a long period has shown that C. verum is not harmful when it is used in the specified conditions. However a long standing excessive use does not exclude concerns with regard to the product safety. Therefore C. verum should not be used during pregnancy and lactation, in cases of fever of unknown origin, stomach or duodenal ulcers, and in patients with an allergy to cinnamon or Peru balsam. No drug interactions are documented. There is no restriction to the duration of use of the herbal substance.
Overall conclusions
Despite of their long tradition, the bark and the essential oil of Cinnamomum verum do not fulfil the requirements of a well-established medicinal use with recognised efficacy. Cinnamon products may be considered as traditional herbal medicinal products on the basis of the long medicinal tradition in the specified conditions.
Benefit-risk assessment
- Quality
The herbal substance or the dried bark of Cinnamomum verum is described in the European Pharmacopoeia. Quality relates to its content of essential oil. There are no cases of contamination or adulteration mentioned in literature. The odour of the herbal substance can be considered as an important tool in the identification process
Conclusion: analytical assessment of the quality of Cinnamomum should be made and the shelf-life of the preparations should be monitored.
- Safety
Cinnamon has been used in Europe since the 13th century. Patients with liver pathology should avoid taking cinnamon because there may be a glutathione depleting action of cinnamaldehyde. No serious adverse effects have been reported. Ingestion of supratherapeutic amounts of cinnamon oil (equivalent to 15 to 20 g bark) can lead to gastro-intestinal irritation and even to central effects like sleepiness, dizziness and a rapid pulse. It should be taken into consideration that all preparations included in the regulatory overview were complex mixtures of which cinnamom oil was one of the components. Administered in such way does make gastro-intestinal irritation less probable.
Abuse of cinnamon oil has been described. Most of the cases reported up to now are not in a medico-pharmaceutical context. Therefore a warning should not be included in the monograph.
There is only limited preclinical information with regard to pregnancy and lactation. High doses were teratogenic for chicken embryos.
Cinnamaldehyde and other cinnamon compounds were mutagenic in the Ames test and the chromosome aberration test. Cinnamaldehyde was genotoxic in the Drosophila test system. Tests with the isolated compounds, however, are of limited value for the possible risks with the herbal substance.
Conclusion: there is no major concern about the safety of Cinnamomum verum bark preparations under conditions given in the monograph. Cinnamon bark and essential oil were not adequately tested on genotoxicity where positive effects were obtained for isolated compounds. Therefore a list entry can not be considered.
- Efficacy
Cinnamon has been used for centuries for dyspeptic complaints. It has to be considered for symptomatic treatment after serious illness is excluded.
Conclusion: see safety conclusion.
See also
- heande:Benefit-risk assessment of plant-based food supplements
- Benefit-risk assessment of food supplements
- Plantlibra
References
- EMA Assessment on cinnamon
- EMA Community herbal monograph on cinnamon
- EMA List of references for Assessment on cinnamon
Related files
<mfanonymousfilelist></mfanonymousfilelist>