State of the art in benefit–risk analysis: Medicines
This page is a nugget.
The page identifier is Op_en5540 | |
|---|---|
| Moderator:EssiV (see all) | |
|
| |
| Upload data
|
Unlike most other pages in Opasnet, the nuggets have predetermined authors, and you cannot freely edit the contents. Note! If you want to protect the nugget you've created from unauthorized editing click here |
This page (including the files available for download at the bottom of this page) contains a draft version of a manuscript, whose final version is published and is available in the Food and Chemical Toxicology 50 (2012) 26–32. If referring to this text in scientific or other official papers, please refer to the published final version as: J.M. Luteijn, B.C. White, H. Gunnlaugsdóttir, F. Holm, N. Kalogeras, O. Leino, S.H. Magnússon, G. Odekerken, M.V. Pohjola, M.J. Tijhuis, J.T. Tuomisto, Ø. Ueland, P.A. McCarron, H. Verhagen: State of the art in benefit–risk analysis: Medicines. Food and Chemical Toxicology 50 (2012) 26–32 doi:10.1016/j.fct.2011.06.008 .
Contents
Title
State of the art in benefit–risk analysis: Medicines
Authors and contact information
- J.M. Luteijn
- (University of Ulster, School of Nursing, United Kingdom, Tel.: +44 (0) 28 90366639, fax: +44 28 90368341,
E-mail address: Luteijn-J@email.ulster.ac.uk)
- B.C. White
- (University of Ulster, Department of Pharmacy & Pharmaceutical Sciences, School of Biomedical Sciences, United Kingdom)
- H. Gunnlaugsdóttir
- (Matís, Icelandic Food and Biotech R&D, Iceland)
- F. Holm
- (FoodGroup Denmark & Nordic NutriScience, Denmark)
- N. Kalogeras
- (Maastricht University, School of Business and Economics, The Netherlands)
- O. Leino
- (National Institute for Health and Welfare (THL), Finland)
- S.H. Magnússon
- (Matís, Icelandic Food and Biotech R&D, Iceland)
- G. Odekerken
- (Maastricht University, School of Business and Economics, The Netherlands)
- M.V. Pohjola
- (National Institute for Health and Welfare (THL), Finland)
- M.J. Tijhuis
- (Maastricht University, School of Business and Economics, The Netherlands)
- (National Institute for Public Health and the Environment (RIVM), The Netherlands)
- J.T. Tuomisto
- (National Institute for Health and Welfare (THL), Finland)
- Ø. Ueland
- (Nofima, Norway)
- P.A. McCarron
- (University of Ulster, School of Nursing, United Kingdom)
- H. Verhagen
- (Maastricht University, NUTRIM School for Nutrition, Toxicology and Metabolism, The Netherlands)
- (National Institute for Public Health and the Environment (RIVM), The Netherlands)
- (University of Ulster, Northern Ireland Centre for Food and Health (NICHE), United Kingdom)
Article info
Article history: Available online 12 June 2011
Abstract
Benefit–risk assessment in medicine has been a valuable tool in the regulation of medicines since the 1960s. Benefit–risk assessment takes place in multiple stages during a medicine’s life-cycle and can be conducted in a variety of ways, using methods ranging from qualitative to quantitative. Each benefit–risk assessment method is subject to its own specific strengths and limitations. Despite its widespread and long-time use, benefit–risk assessment in medicine is subject to debate and suffers from a number of limitations and is currently still under development.
This state of the art review paper will discuss the various aspects and approaches to benefit–risk assessment in medicine in a chronological pathway. The review will discuss all types of benefit–risk assessment a medicinal product will undergo during its lifecycle, from Phase I clinical trials to post-marketing surveillance and health technology assessment for inclusion in public formularies. The benefit– risk profile of a drug is dynamic and differs for different indications and patient groups. In the end of this review we conclude benefit–risk analysis in medicine is a developed practice that is subject to continuous improvement and modernisation. Improvement not only in methodology, but also in cooperation between organizations can improve benefit–risk assessment.
Abbreviations
ADR, adverse drug reaction; BR, benefit–risk; CHMP, Committee for Medicinal Products for Human use; EC, European Commission; EMA, European Medicines Agency; EU, European Union; EudraVigilance, European Union Drug Regulating Authorities Pharmacovigilance; FDA, Food and Drug Administration; MHRA, Medicines and Healthcare Products Regulatory Agency; NHS, National Health Service; NICE, National Institute for Clinical Excellence; PMS, post-marketing surveillance; PROTECT, Pharmacoepidemiological Research on Outcomes of Therapeutics by a European Consortium; QALY, quality-adjusted life year; TMF, trial master file; TURBO, Transparent Uniform Risk Benefit Overview; UK, United Kingdom; WHO, World Health Organization.
Keywords
Benefit–risk assessment, Health technology assessment, Pharmacovigilance, Drug approval/methods, Risk assessment
Introduction
The regulation of medicines was transformed in the wake of the thalidomide disaster in the 1960s (Botting, 2002). Prior to this event there was limited control on the safety and efficacy of medicines, including no demands on efficacy demonstration. Currently the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK, the European Medicines Agency (EMA) in the European Union and the Food and Drug Administration (FDA) in the United States, are amongst the various bodies responsible for promoting and protecting public health by ensuring the availability of safe and effective medicines. These agencies are responsible for the evaluation, supervision and pharmacovigilance of medicinal products throughout the lifecycle of the medicine.
Benefit–risk (BR) analysis of medicines is a complex process that requires evaluation of a large amount of relevant data. Clinical trials are used to evaluate safety of a new drug in healthy volunteers or patients, and/or to assess treatment benefit in patients with a specific clinical condition. The majority of clinical trials are sponsored by pharmaceutical companies developing new drugs. Although pre-marketing clinical trials lead to valuable safety information, there are several limitations. Many clinical trials only have a relatively small and carefully selected population which is hardly indicative of the much larger and diverse expanse of patients including those with co-morbid conditions and those being treated with concomitant medications. Therefore, post marketing safety data collection and clinical risk assessment based on observational data are critical for evaluating and characterising a product’s risk profile and for making informed decisions on risk minimisation (Lu, 2010). Recently, drugs such as rimonabant, cisapride and rofecoxib have been withdrawn from the market after post-marketing studies raised safety concerns. These withdrawals highlight the value of post-marketing studies and the dynamic balance of perceived benefit and perceived risk. This paper presents an overview of existing approaches to BR assessment in medicines for human use by the various regulatory bodies at each of the following stages: marketing authorisation procedure, the health technology assessment and the post marketing surveillance. This paper will mainly discuss the European perspectives and approaches to BR assessment in medicines.
Key terms
The MHRA defines a medicinal product as: ‘‘(A) any substance or combination of substances presented as having properties for treating or preventing disease in human beings. (B) Any substance or any combination of substances which may be used in or administered to human beings with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action or to make a medical diagnosis’’.
There is no standard, widely acknowledged definition of the terms benefit and risk as applied to medicine and particularly medicinal products. The EMA’s benefit–risk methodology project will come up with a definition of its own for benefit and risk by the end of 2011 (EMA, 2010a).
Efficacy refers to the probability of expected benefit to individuals in a defined population from a medical technology applied for a given medical problem under ideal conditions of use, while effectiveness refers to the probability of expected benefit to individuals in a defined population from a medical technology applied for a given medical problem under ordinary conditions of use (Brook and Lohr, 1985).
An adverse drug reaction or ADR as defined by Edwards and Aronson (2000) is ‘‘an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product (Edwards and Aronson, 2000)’’.
Pharmacovigilance is the science and activities relating to detection, assessment, understanding and prevention of adverse effects or any other drug-related problem that may cause shortand long-term side effects. The word is derived from the Greek pharmakon – remedy/recipe, cure or drug, and the Latin vigilare – to be awake or alert, to keep watch.
Drug discovery and development
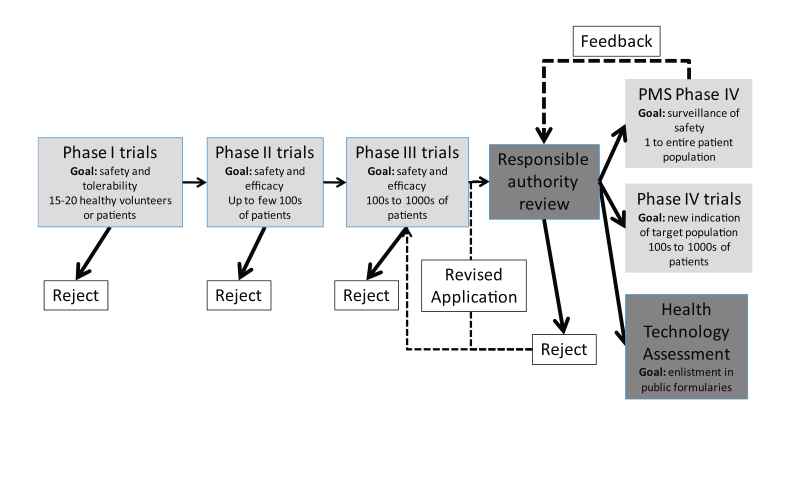
In the UK, the MHRA grants authorisation for a clinical trial of a medicinal product to be carried out in healthy volunteers. Trials need to be conducted in accordance with Good Clinical Practice guidelines as outlined in articles 2–5 in the European Union (EU) Directive 2005/28/EC and Schedule 1, Part 2 of the UK Clinical Trial Regulations (EC, 2001). Good clinical practice is a set of internationally recognised ethical and scientific quality requirements which must be observed for designing, conducting, recording and reporting clinical trials that involve the participation of human subjects. Trials are classified according to the four clinical phases of development of a particular drug (Phases I–IV, see Fig. 1). BR assessment will be performed by the manufacturer using incremental knowledge throughout these clinical trials. Most drugs entering Phase I trials will never reach Phase IV trials, but rather get rejected by the manufacturer after BR assessment during Phase I–III trials indicates an unfavourable BR profile. The perceived BR profile of a drug is dynamic and will change with every piece of evidence gathered during these trials. Phase I–III trials involve an increasing and considerable financial investment by the manufacturer, and trials will only be continued if the manufacturer feels the drug stands a chance to successfully gain marketing authorisation. Phase I–III trials take place under controlled, ideal circumstances. As a result, benefit is represented by efficacy, rather than effectiveness.
Phase I trials emphasize on risk by testing the tolerability and safety of a drug in healthy volunteers or patients unresponsive to usual therapies. This is achieved by assessing pharmacodynamic and pharmacokinetic parameters, particularly with respect to immediate short-term safety of higher doses. During Phase II clinical trials, dose–response curves in patients and efficacy of the drug seen in patients with a particular disease are examined. Phase III clinical trials are where the drug is tested in a controlled fashion against a placebo or standard therapy; this is where the medicinal product will either make or break its reputation with respect to safety and efficacy. A positive study in Phase III is also known as a landmark study through which it might gain a licence to be prescribed for a specific disease. There has been criticism on the design of preapproval trials being designed for rapid approval and not for the generation of scientific knowledge (Avorn, 2006). For example, women and elderly persons are systematically excluded from clinical trials in cardiovascular disease (Lee et al., 2001) and important co-morbidities are underrepresented in clinical trials. Benefit and sometimes risk in clinical trials are captured by clinical trial endpoints. Clinical trial endpoints are often ‘‘surrogate’’ endpoints, for example blood pressure, instead of hard endpoints like survival. Surrogate endpoints have certain advantages, such as requiring smaller sample sizes and allowing for faster evaluation than hard endpoints. Surrogate endpoints, however, can be misleading because of underlying assumptions in regard to hard endpoint health benefits, which may or may not be true (Psaty et al., 1999). In addition it has been demonstrated that studies sponsored by pharmaceutical companies are four times more likely to favour the product of the sponsor than studies with other sponsors (Lexchin et al., 2003). Possible sources of this bias suggested by Lexchin et al. (2003) include inappropriate comparators and publication bias. Publication bias meaning studies containing positive outcomes are more likely to be offered for publication in addition to be more likely to be accepted for publication than studies containing negative outcomes. Once Phase III clinical trials results are deemed satisfactory by the manufacturer, a regulatory submission can be made to regulatory authorities for marketing authorisation. This submission includes results of clinical trials, but also other matters like a risk management plan and can be rejected on other matters than Phase I–III trial results. Once marketing authorisation has been granted, clinical trials enter Phase IV and the marketing authorisation holder can apply for enlisting in public formularies (enlistment in health insurance packages) by means of health technology assessment (see Section 5). A study in Phase IV is often called a post marketing study as the drug has been granted a marketing authorisation. Two types of Phase IV studies exist: post-marketing surveillance (PMS) studies which can be undertaken by responsible authorities, independent researchers or the marketing authorisation holder and studies undertaken by the marketing authorisation holder for finding a new market (either a new indication or new patient population). Post marketing study populations can range from single case reports to the entire patient population.
PMS studies are crucial for gathering additional safety information from a larger group of patients in order to understand the long-term safety of the drug, appreciate drug interactions and safety in rare patient populations. PMS studies play a role in BR monitoring (see Section 6).
Every trial is legally required to have a trial master file (TMF), a sponsor and a European Union Drug Regulating Authorities Clinical Trials (EudraCT) number. The TMF will contain all the essential documents for all stages of the trial and is held at the principal site chief investigators office although it should be accessible at all participating sites. Trials evaluating commercial products will often be sponsored by the corresponding pharmaceutical company, whereas publicly funded trials will be sponsored by the UK-National Health Service (NHS) or an independent research institution. EudraCT is a database of all clinical trials in the EU from 2004. The sponsor of the clinical trial is required to report all relevant information regarding suspected serious ADRs that are fatal or life threatening to the MHRA within a week and all other adverse reactions or events within a fortnight. Yearly reporting of all suspected serious ADRs that have occurred and a report on the subjects’ safety are required as well (Directive 2001/20/EC). Currently ADRs are reported to several authorities if a product is authorised in more than one member state which may ultimately lead to duplicative assessments. To streamline this process it is proposed that all adverse drug reaction data will be reported directly to the EudraVigilance database. This would help identify early safety signals from Clinical trials across the European Union.
Marketing authorisation procedures in the EU
The overall benefit–risk (BR) profile of the drug plays an important role in marketing authorisation procedures. Risk is represented by unintended side effects, through unintended channels of biologic influence. Benefit is represented by the health gain realised by the intended mechanism of action of the medicine. As a result, the context of the severity of the disease treated is of large influence on the benefit, and therefore the overall BR balance. As a consequence, the BR balance will vary for different indications of the same drug and a drug might gain marketing authorisation for one indication, but not for another. The benefit–risk profile of a drug can be assessed using a number of diverse BR assessments, each with specific flaws and strengths.
BR assessment of medicinal products ranges from qualitative BR assessment to quantitative BR analysis. An example of a quantitative BR analysis is the Number Needed to Treat analysis, which calculates the number of individuals that need to be treated for a number of years in order to prevent a single case of negative outcome such as disease. The opposite of Number Needed to Treat, Number Needed to Harm, calculates the number of individuals that need to be treated to cause a single case of the ADR of interest. A recent report from the EMAs BR methodology project identified 3 systematic reviews on quantitative BR analyses methodologies for a combined total of 18 different quantitative BR analysis methodologies (EMA, 2010b). On the other hand, expert judgement is a qualitative form of BR analysis in medicines. There exists a range of BR assessment methods somewhere in between qualitative and quantitative based on indexing and scoring evidence. An example of this is the TURBO model (CIOMS, 1998) which attempts to quantify benefits and risks of a medicine for a given indication, both factors graphically displayed in a TURBO diagram.
Some well known BR assessment models were discussed by the Committee for Medicinal Products for Human use CHMP (2007). The CHMP recommended use of mainly qualitative techniques mainly because numerical models can convey a misleading feeling of precision and my shift the focus on overall numerical summaries at the expense of information on the qualitative differences (CHMP, 2007). Because each BR assessment method carries its specific strengths and limitations, it has been recommended to make use of several BR assessment methods over different patient populations and treatment indications in a BR over different patient populations and treatment indications in a BR analysis (Guo et al., 2010).
BR assessment is performed in drug marketing authorisation procedures by national responsible authorities. Within the European Union, marketing authorisation can be applied for either by the centralised procedure (or community authorisation procedure), the decentralised procedure, the mutual recognition procedure or national procedures (EC, 2005). In the national procedure, the marketing authorisation application is for a single country and progresses according to national guidelines in that specific country. Centralised, decentralised and mutual recognition procedures can be used to apply for a marketing authorisation in several European countries simultaneously. For decentralised procedures, mutual recognition procedures and national procedures, the authorities for member states are the responsible authority, while for centralised procedures, the European Commission (EC) is the competent authority (EC, 2005). Centralised procedures are facilitated via the EMA In centralised procedures the BR analysis is carried out by staff of one or two member states, referred to as the rapporteur and the co-rapporteur who report back to the Committee for Medicinal Products for Human use (CHMP). The CHMP advises the EC on the matter and if successful, the EC grants the marketing authorisation. The centralised procedure is compulsory for human medicines that are derived from biotechnological processes, for treatment of rare disorders (orphan medicines) or for treatment of HIV/AIDS, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions, while the decentralised procedure is used for medicinal products that do not fall under the scope of the centralised procedure (EMA, 2010). The mutual recognition procedure is used for medicinal products that are already authorised via a national procedure. via the mutual recognition procedure, marketing authorisation can be sought for a nationally authorised medicinal product in a European country, in other European countries. In this procedure, the countries concerned agree to recognise the validity of the original, national marketing authorisation (EC, 2005). It is important to notice that in all the marketing authorisation procedures, the BR analysis is carried out by staff of national responsible authorities and not the EMA. In addition, the EMA does not authorise marketing authorisation procedures, the EC does so for centralised procedures and national competent authorities does so for all other procedures.
In Europe, assessment reports on clinical, chemical-pharmacological and toxicological characteristics are completed by the national responsible authority before the national responsible authority schedules an expert meeting to discuss and decide on the BR profile of the candidate-drug and the drug-approval process. Depending on the BR profile, the marketing authorisation can be granted, granted with conditions or can be rejected. Additional conditions usually have to do with limiting the indication of the medicinal product or implementing a stringent risk management programme and in some cases risk minimisation activities (EC, 2008).
In drug marketing authorisation procedures, BR assessment is used as a tool to formulate an expert opinion on the authorisation procedure. In addition to the BR profile, some other factors should be considered as well. Internal factors in authorisation procedures are the capability of healthcare systems to optimise benefit and minimise known risks and medication errors (Honig, 2007). An important external factor is the community acceptance of risk, which is currently shifting to a more risk-averse mentality (Walker et al., 2009). No consensus on choice of BR assessment method in drug authorisation procedures has been achieved yet. In Europe, detailed guidance on the principles and methodology for BR assessment is currently lacking (CHMP, 2008). Stakeholders involved in drug marketing authorisation procedures are currently working towards standardised BR procedures and analyses. In a recent BR analysis assessment report by the CHMP, the CHMP argued in favour of interaction with relevant stakeholders, both on a European and international level (CHMP, 2008).
Health technology assessment
HTA in public healthcare
Once a drug has been granted marketing authorisation, applications can be made for a drug to be reimbursed by third party payers such as national health services or social security agencies via health technology assessment (HTA). For many drugs, reimbursement by third party payers is crucial to patient availability, undermining the importance of winning marketing authorisation approval and complicating the route to the marketplace for new drugs (Eichler et al., 2010). In order for a drug to qualify for enlistment, cost-effectiveness needs to be demonstrated using HTA methodology. In contrast to the BR analysis which focuses on safety and efficacy, HTA focuses on the clinical and economic value of a health intervention, often relative to the current best option available using comparative effectiveness research. The clinical value of a health intervention in HTA involves subtracting risks from benefits and essentially is the equivalent to an assessment of the BR profile of a health intervention (Garrison, 2010). HTA addresses the question whether the new technology is worth the (additional) costs relative to the best option available. HTA attempts to calculate the ratio between risk (or costs) and benefit of a health technology, expressing the change in benefit the health technology would cause per unit of costs relative to alternative health technologies. The term costs can include indirect costs (e.g. loss of productivity by the victim of a stroke) in addition to direct costs (e.g. production costs of a medicine). For HTA, whether or not to include indirect costs depends on the payer’s perspective of the analysis. For national agencies, the community perspective will be used, meaning indirect costs are included in the analysis. However, from for example a hospital’s perspective, indirect costs will not be relevant and therefore not be included in the HTA.
National agencies, such as the National Institute for Clinical Excellence (NICE) in England and Wales and the Health Care Insurance Board (CVZ) in the Netherlands, conduct HTAs and decide on whether or not to enlist the assessed drug in public formularies. HTA by these agencies can lead to three outcomes: list, list with criteria or do not list (Dakin et al., 2006). List with criteria might involve recommendations for a different, usually more restricted patient population. NICE decisions have been demonstrated to be of considerable influence on National Health Service resource allocation, patient access to new technologies and pharmaceutical sales and profit (Raftery, 2001; Howard and Harrison, 2004). Considerable differences exist between national HTA agencies, their origin and their jurisprudences. Some agencies, like NICE are linked to the payer in this specific case to the National Health System, others are part of a professional society, whereas in the USA, HTA can be carried out by commercial stakeholders. An example of different jurisprudences is given by the Australian Pharmaceutical Benefits Advisory Committee, which can engage in price negotiations. This is in contrast to other agencies like the NICE that cannot engage in price negotiations. A recent retrospective analysis of three such agencies has discovered differences in approval rates of drugs submitted for listing, ranging from 49.6% for the Canadian Common Drug Review to 87.4% for NICE (Clement et al., 2009). The high percentage of listings by NICE includes a high number of ‘‘list with criteria’’ recommendations, in contrast to the listings by the other two agencies. We believe the differences between HTA agencies reflect a lack of consensus on who should appreciate benefit– risk evidence in medicine and the jurisprudences of those who do. HTA agencies are not involved in setting the health system budget, and therefore have to work with the available resources allocated. The main role of HTA agencies therefore is to get the most health gain out of the limited resources available for healthcare. HTA agencies have been criticised for their execution of this role and NICE is considered a controversial agency by some (Birch and Gafni, 2007;Carroll, 2009). Different viewpoints of HTA agencies and responsible authorities can discrepancies in decision making adding an extra layer of complexity to the route of new drugs to the marketplace (Eichler et al., 2010).
HTA methodology
HTA can be carried out on a single intervention (single technology assessment), or on multiple interventions at the same time, such as a group of drugs (multiple technology assessment). HTA methodology differs amongst organizations conducting HTAs (Drummond et al., 2008). The methodology used by NICE can be divided into three distinct phases: scoping, assessment and appraisal (NICE, 2008). During the scoping phase, the research questions and the scope of the technology appraisal are formulated. Important contextual factors such as the population and the comparator technologies are formulated as well. The assessment phase consists of systematically reviewing available evidence and an economic evaluation of the technology assessed. During the appraisal, an appraisal decision is made on base of the assessment and additional information supplied by stakeholders. The economic evaluation involves a cost-effectiveness analysis and the outcome should have an impact on survival or health-related quality of life and be able to be translated in terms of quality-adjusted life years (QALY). Both benefit and risk are expressed in the same index term after which risk is subtracted from benefit after which the costs per QALY can be calculated. Both HTA and quantitative BR analysis aim at calculating the net benefit of health interventions. It has been argued that the modelling methods and framework used in HTA assessment can provide a promising, more structured approach in BR assessment by responsible authorities (Garrison et al., 2007). The costeffectiveness threshold for listing in public formularies used by NICE has never been explicitly stated, but is believed to be somewhere around the £20,000–30,000 per QALY (Carroll, 2009). Economic analyses usually incorporate a reference standard, for comparison and a sensitivity analysis. A sensitivity analysis can be either univariate or multivariate and is conducted to test the impact of varying important and/or uncertain parameters on the outcome of the economic analysis. In univariate sensitivity analyses, a single parameter is varied, while in multivariate sensitivity analyses the impact of varying multiple parameters at the same time is tested. Sensitivity analyses’ intervals are usually varied on the scale of a confidence interval provided by the source of the parameter or by varying between the different estimates of the parameter as estimated by different sources.
Benefit–risk monitoring
Why benefit–risk monitoring?
With the marketing authorisation of a drug, the drug appraisal process enters a new phase: the PMS phase, or Phase IV. PMS is performed by responsible authorities, marketing authorisation holders and independent researchers in order to collect data on ADRs. Pre-marketing knowledge will lead to a relatively limited view of the risk profile of a drug due to several limitations, which include the small number of subjects in clinical trials, a restricted population in terms of age, gender and ethnicity, restricted comedication and co-morbidity, a short duration of exposure and follow up and statistical problems with assessing multiple outcomes (EC, 2008). Off-label use, drug–drug interactions and non-compliance might also lead to new ADRs unidentifiable in pre-marketing trials. A number of ADRs can be expected by marketing experience from related drugs (if any), animal studies and clinical trials, but especially rare ADRs or those occurring in specific patient populations are unlikely to be discovered before marketing authorisation. It should be noted that PMS focuses solely on monitoring the risks of marketed drugs, not the benefits, though the marketing authorisation holder might conduct trials to explore benefits in different patient populations, or for different indications than currently being authorised. ADRs discovered during PMS demand reassessment of the BR profile of a drug by BR reassessment. BR reassessment can lead to possible suspension of marketing licence or (adjustment of) risk management and/or risk minimisation activities. PMS is also used for evaluating the effectiveness of existing risk management activities. A good example of modification of risk management activities after evaluation by PMS is the risk minimisation programme for preventing birth defects caused by isotretinoin in the USA. This programme has been modified 4 times between 1982 and 2006, each time becoming more stringent after PMS demonstrated the lack of effectiveness of the existing programme (Abroms et al., 2006).
The benefit–risk monitoring process
A large number of stakeholders are involved in PMS, including patients, healthcare professionals, the marketing authorisation holder and responsible authorities. In Europe, the guidelines for pharmacovigilance, which includes post-marketing surveillance, are presented in volume 9A of the rules governing medicinal products in the European Union (EC, 2008). During the marketing authorisation procedure, a risk management plan is submitted by the candidate marketing authorisation holder, tailored to the known and expected risks of the drug. This risk management programme is thoroughly reviewed by the responsible authorities. The EU-risk management programme consists of two parts; part 1 containing a safety specification and a pharmacovigilance plan and part 2 containing the evaluation for the need of risk minimisation activities and, if required, a risk minimisation plan. In 2001, the European Union Drug Regulating Authorities Pharmacovigilance (EudraVigilance) data processing network was developed in order to aid the reporting, evaluation and processing of suspected ADRs within the EU (http://eudravigilance.emea.europa.eu/human/index.asp). All national authorities involved in the EMA can access the database; it is meant for national responsible authorities to share information and benefit from another’s work. PMS and pharmacovigilance in general has been developed since the thalidomide tragedy in the 1960s, but the current system is not perfect and a lot of development is currently going on in the post-marketing surveillance. A good example of this is the withdrawal of Rofexocib (Vioxx) in September 2004 (Avorn, 2006;Juni et al., 2004). Vioxx was shown to almost double the risk of myocardial infarction and stroke compared to both placebo and alternative treatment (naproxen). Signals for this were apparent before Vioxx was approved, but not followed up, leading to exposure of millions of patients. PMS has changed from being passive to a more proactive approach by risk management and using risk minimisation plans. Recent European projects to improve the PMS include the EU-ADR project, which attempts at mining electronic healthcare records from different sources for signals (Trifiro et al., 2009). The European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP) which attempts at facilitating the conduct of multi-centre post-authorisation studies focussing on BR profiles and safety and the PROTECT project (http://www.imiprotect. eu/), coordinated by the EMA, which aims at addressing limitations in current methods, including BR assessment, in the fields of pharmacoepidemiology and pharmacovigilance . In the EU, the manufacturer is required to submit Periodic Safety Update Reports at specific time intervals, depending on the post-marketing experience and the safety profile of the drug. The major challenges of post-marketing surveillance are dealing with uncertainty and acting fast. Once a signal of a possible ADR arises, the decision has to be made whether to act, or to gather additional evidence. On occasions, alerts from ADRs are not acted upon fast enough and patients experience exacerbated adverse effects. Statistical power takes time to gather. In the case of unexpected ADRs, in particular, initial evidence from PMS is usually scarce and limited to the low level of evidence provided by single case reports or case series. Suspending or limiting the marketing authorisation of the drug will lead to denying patients the drug benefits, while every day of additional investigation required might cause additional ADRs to happen.
When ADRs are identified or suspected, healthcare professionals should be informed via risk communication. Responsible authorities or the marketing authorisation holder can send a direct healthcare professional communication to healthcare professionals to inform them on market suspensions, important changes to the product information leaflet or other situations relevant to the safe and effective use of the drug (EC, 2008). The goal of direct healthcare professional communications is to allow the healthcare professional to include this new information in his professional judgement when prescribing to patients. Direct healthcare professional communications are used in case of urgency. In cases of less urgency, the product package or product information leaflet will be updated without direct communication.
The present and future of benefit–risk monitoring
BR analysis in drugs is older and better developed than BR in other fields of research such as food and nutrition and environmental health, but there still is much to be improved upon. Initiatives like the EudraVigilance database and the EU-ADR project might lead to additional power to detect and assess ADRs faster, enabling faster and more refined BR profile estimates of marketed drugs. More refined BR profile estimates will allow for improved comparative effectiveness analyses providing responsible authorities with valuable data for evidence based decision making. A new round of European legislation for pharmacovigilance is currently being prepared (Moore and Begaud, 2010). This new legislation and the European PROTECT project will further modernise and improve the fields of pharmacoepidemiology and pharmacovigilance.
Conclusion
BR analysis in drugs is a fast developing practice, both in terms of methodology and legislation. A large number of BR assessment methods are available, each offering its own strengths and limitations. Due to each method suffering from its own specific limitations, use of multiple BR assessment methods is encouraged for BR assessment. No one size fits all approach exists for BR assessment in medicine. Choice of BR assessment method depends on the context of the BR assessment, including its indication, patient groups and the stage in the regulatory process. There is room for improvement and improved cooperation between responsible authorities and between HTA agencies and responsible authorities would be of benefit.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The preparation of this manuscript was funded through Safefoodera Project BEPRARIBEAN (Project ID 08192) by the Dutch Food and Consumer Product Safety Authority (VWA), the Research Council of Norway (RCN) and the Nordic Council of Ministers (NCM) and supported by Matís, The National Institute for Health and Welfare (THL), the University of Ulster and the National Institute for Public Health and the Environment (RIVM).
References
Abroms, L., Maibach, E., Lyon-Daniel, K., Feldman, S.R., 2006. What is the best approach to reducing birth defects associated with isotretinoin? PLoS Med. 3, e483. doi:10.1371/journal.pmed.0030483 .
Avorn, J., 2006. Evaluating drug effects in the post-Vioxx world: there must be a better way. Circulation 113, 2173–2176. doi:10.1161/CIRCULATIONAHA.106.625749 .
Birch, S., Gafni, A., 2007. Economists’ dream or nightmare? Maximizing health gains from available resources using the NICE guidelines. Health. Econ. Policy. Law. 2, 193–202. doi:10.1017/S1744133107004057 .
Botting, J., 2002. The history of thalidomide. Drug News Perspect. 15, 604–611.
Brook, R.H., Lohr, K.N., 1985. Efficacy, effectiveness, variations, and quality.
Boundary-crossing Res. Med. Care 23, 710–722.
Carroll, S., 2009. Is there anything nicer than NICE? A question the conservative shadow health team is right to ask. Value Health. doi:10.1111/j.15244733.2009.00546.x .
Committee for Medicinal Products for Human use (CHMP), 2007. Report of the CHMP working group on benefit–risk assessment models and methods. Doc. Ref. EMEA/CHMP/15404/2007.
Committee for Medicinal Products for Human use (CHMP), 2008. Reflection paper on benefit–risk assessment methods in the context of the evaluation of marketing authorisation applications of medicinal products for human use. Doc. Ref. EMEA/CHMP/15404/2007.
Council for International Organizations of Medical Sciences (CIOMS), 1998. Benefit– Risk Balance for Marketed Drugs: Evaluating Safety Signals. Report of CIOMS Working Group IV. Available from <http://www.cioms.ch/publications/g4-benefit-risk.pdf>.
Clement, F.M., Harris, A., Li, J.J., Yong, K., Lee, K.M., Manns, B.J., 2009. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA 302, 1437–1443. doi:10.1001/jama.2009.1409 .
Dakin, H.A., Devlin, N.J., Odeyemi, I.A., 2006. ‘‘Yes’’, ‘‘No’’ or ‘‘Yes, but’’? Multinomial modelling of NICE decision-making. Health Policy 77, 352–367. doi:10.1016/j.healthpol.2005.08.008 .
Drummond, M.F., Schwartz, J.S., Jonsson, B., Luce, B.R., Neumann, P.J., Siebert, U., Sullivan, S.D., 2008. Key principles for the improved conduct of health technology assessments for resource allocation decisions. Int. J. Technol. Assess. Health Care 24, 244–258. doi:10.1017/S0266462308080343 (discussion 362–8).
European Commission (EC), 2001. Directive 2001/83/EC of the European Parliament and the Council of 6 November 2001 on the community code relating to medicinal products for human use. Official Journal L – 311, 28/11/2001, 67–128.
European Commission (EC), 2005. Volume 2A – Procedures for Marketing Authorisation.
European Commission (EC), 2008. Volume 9A – The Rules Governing Medicines Products in the European Union: Guidelines on Pharmacovigilance for Medicinal Products for Human Use.
Edwards, I.R., Aronson, J.K., 2000. Adverse drug reactions: definitions, diagnosis, and management. Lancet 356, 1255–1259. doi:10.1016/S0140-6736(00)02799-9 .
Eichler, H.G., Bloechl-Daum, B., Abadie, E., Barnett, D., Konig, F., Pearson, S., 2010. Relative efficacy of drugs: an emerging issue between regulatory agencies and third-party payers. Nat. Rev. Drug Discov. 9, 277–291. doi:10.1038/nrd3079 .
European Medicines Agency (EMA), 2010. Authorisation Procedures in the European Union.
European Medicines Agency (EMA), 2010a. European Medicines Agency Benefit– Risk methodology project. Doc. Ref. EMA/213482/2010.
European Medicines Agency (EMA), 2010b. Benefit–risk methodology project Work package 2 report: Applicability of current tools and processes for regulatory benefit–risk assessment. Available from <http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/10/WC500097750.pdf>.
Garrison, L.P., 2010. Regulatory benefit–risk assessment and comparative effectiveness research: strangers, bedfellows or strange bedfellows? Pharmacoeconomics 28, 855–865. doi:10.2165/11538640-000000000-00000 .
Garrison Jr., L.P., Towse, A., Bresnahan, B.W., 2007. Assessing a structured, quantitative health outcomes approach to drug risk-benefit analysis. Health. Aff. (Millwood) 26, 684–695. doi:10.1377/hlthaff.26.3.684 .
Guo, J.J., Pandey, S., Doyle, J., Bian, B., Lis, Y., Raisch, D.W., 2010. A review of quantitative risk-benefit methodologies for assessing drug safety and efficacyreport of the ISPOR risk-benefit management working group. Value Health. doi:10.1111/j.1524-4733.2010.00725 .
Honig, P., 2007. Benefit and risk assessment in drug development and utilization: a role for clinical pharmacology. Clin. Pharmacol. Ther. 82, 109–112. doi:10.1038/sj.clpt.6100277 .
Howard, S., Harrison L., 2004. NICE guidance implementation tracking: data sources, methodology and results. A Report Commissioned by the NICE. Available from <http://www.nice.org.uk/>. Bicester: Abacus International.
Juni, P., Nartey, L., Reichenbach, S., Sterchi, R., Dieppe, P.A., Egger, M., 2004. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 364, 2021–2029. doi:10.1016/S0140-6736(04)17514-4 .
Lee, P.Y., Alexander, K.P., Hammill, B.G., Pasquali, S.K., Peterson, E.D., 2001. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA 286, 708–713.
Lexchin, J., Bero, L.A., Djulbegovic, B., Clark, O., 2003. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 326, 1167–1170. doi:10.1136/bmj.326.7400.1167 .
Lu, Z., 2010. Technical challenges in designing post-marketing eCRFs to address clinical safety and pharmacovigilance needs. Contemp. Clin. Trials 31, 108–118. doi:10.1016/j.cct.2009.11.004 .
Moore, N., Begaud, B., 2010. Improving pharmacovigilance in Europe. BMJ 340, c1694. National Institute for Health and Clinical Excellence (NICE), 2008. Guide to the Methods of Technology Appraisal. Available from <http://www.nice.org.uk/>.
Psaty, B.M., Weiss, N.S., Furberg, C.D., Koepsell, T.D., Siscovick, D.S., Rosendaal, F.R., Smith, N.L., Heckbert, S.R., Kaplan, R.C., Lin, D., Fleming, T.R., Wagner, E.H., 1999. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA 282, 786–790.
Raftery, J., 2001. NICE: faster access to modern treatments? Analysis of guidance on health technologies. BMJ 323, 1300–1303.
Trifiro, G., Fourrier-Reglat, A., Sturkenboom, M.C., Diaz Acedo, C., Van Der Lei, J., Group, E.U.-A.D.R., 2009. The EU-ADR project: preliminary results and perspective. Stud. Health Technol. Inform. 148, 43–49.
Walker, S., McAuslane, N., Liberti, L., Salek, S., 2009. Measuring benefit and balancing risk: strategies for the benefit–risk assessment of new medicines in a risk-averse environment. Clin. Pharmacol. Ther. 85, 241–246. doi:10.1038/clpt.2008.277 .
Related files
<mfanonymousfilelist></mfanonymousfilelist>