State of the art in benefit–risk analysis: Introduction
This page is a nugget.
The page identifier is Op_en5659 | |
|---|---|
| Moderator:Essi Vuorinen (see all) | |
|
| |
| Upload data
|
Unlike most other pages in Opasnet, the nuggets have predetermined authors, and you cannot freely edit the contents. Note! If you want to protect the nugget you've created from unauthorized editing click here |
This page (including the files available for download at the bottom of this page) contains a draft version of a manuscript, whose final version is published and is available in the Food and Chemical Toxicology 50 (2012). If referring to this text in scientific or other official papers, please refer to the published final version as: H. Verhagen, M.J. Tijhuis, H. Gunnlaugsdottir, N. Kalogeras, O. Leino, J.M. Luteijn, S.H. Magnússon, G. Odekerken, M.V. Pohjola, J.T. Tuomisto, Ø. Ueland, B.C. White, F. Holm: State of the art in benefit–risk analysis: Introduction. Food and Chemical Toxicology 50 (2012) 2–4 doi:10.1016/j.fct.2011.06.007 .
Contents
Title
Editing State of the art in benefit–risk analysis: Introduction
Authors and contact information
- H. Verhagen, correspondence author
- (E-mail address: hans.verhagen@rivm.nl)
- (National Institute for Public Health and the Environment (RIVM), PO Box 1, 3720 BA Bilthoven, The Netherlands)
- (Maastricht University, NUTRIM School for Nutrition, Toxicology and Metabolism, PO Box 616, 6200 MD Maastricht, The Netherlands)
- (University of Ulster, Northern Ireland Centre for Food and Health (NICHE), Cromore Road, Coleraine, BT52 1SA Northern Ireland, United Kingdom)
- M.J. Tijhuis
- (National Institute for Public Health and the Environment (RIVM), PO Box 1, 3720 BA Bilthoven, The Netherlands)
- (Maastricht University, School of Business and Economics, PO Box 616, 6200 MD Maastricht, The Netherlands)
- H. Gunnlaugsdóttir
- (Matís, Icelandic Food and Biotech R&D, Vínlandsleið 12, 113 Reykjavík, Iceland)
- N. Kalogeras
- (Maastricht University, School of Business and Economics, PO Box 616, 6200 MD Maastricht, The Netherlands)
- O. Leino
- (National Institute for Health and Welfare (THL), PO Box 95, FI-70701 Kuopio, Finland)
- J.M. Luteijn
- (University of Ulster, School of Nursing, Shore Road, Newtownabbey (Jordanstown), BT37 0QB Northern Ireland, United Kingdom)
- S.H. Magnússon
- (Matís, Icelandic Food and Biotech R&D, Vínlandsleið 12, 113 Reykjavík, Iceland)
- G. Odekerken
- (Maastricht University, School of Business and Economics, PO Box 616, 6200 MD Maastricht, The Netherlands)
- M.V. Pohjola
- (National Institute for Health and Welfare (THL), PO Box 95, FI-70701 Kuopio, Finland)
- J.T. Tuomisto
- (National Institute for Health and Welfare (THL), PO Box 95, FI-70701 Kuopio, Finland)
- Ø. Ueland
- (Nofima, Osloveien 1, N-1430 Ås, Norway)
- B.C. White
- (University of Ulster, Dept. of Pharmacy and Pharmaceutical Sciences, School of Biomedical Sciences, Cromore Road, Coleraine, BT52 1SA Northern Ireland, United Kingdom)
- F. Holm
- (FoodGroup Denmark and Nordic NutriScience, Rugaardsvej 14, A2 Rugaard, DK-8400 Ebeltoft, Denmark)
Article info
Article history: Available online 12 June 2011
Abstract
Risk-taking is normal in everyday life if there are associated (perceived) benefits. Benefit-Risk Analysis (BRA) compares the risk of a situation to its related benefits and addresses the acceptability of the risk. Over the past years BRA in relation to food and food ingredients has gained attention. Food, and even the same food ingredient, may confer both beneficial and adverse effects. Measures directed at food safety may lead to suboptimal or insufficient levels of ingredients from a benefit perspective. In BRA, benefits and risks of food (ingredients) are assessed in one go and may conditionally be expressed into one currency. This allows the comparison of adverse and beneficial effects to be qualitative and quantitative. A BRA should help policy-makers to make more informed and balanced benefit-risk management decisions. Not allowing food benefits to occur in order to guarantee food safety is a risk management decision much the same as accepting some risk in order to achieve more benefits. BRA in food and nutrition is making progress, but difficulties remain. The field may benefit from looking across its borders to learn from other research areas. The BEPRARIBEAN project (Best Practices for Risk-Benefit Analysis: experience from out of food into food; http://en.opasnet.org/w/Bepraribean) aims to do so, by working together with Medicines, Food Microbiology, Environmental Health, Economics & Marketing-Finance and Consumer Perception. All perspectives are reviewed and subsequently integrated to identify opportunities for further development of BRA for food and food ingredients. Interesting issues that emerge are the varying degrees of risk that are deemed acceptable within the areas and the trend towards more open and participatory BRA processes. A set of 6 ‘state of the art’ papers covering the above areas and a paper integrating the separate (re)views are published in this volume.
Keywords
Benefit-risk, Best practice, BEPRARIBEAN
Introduction
Risk-taking is normal in everyday life if there are associated (perceived) benefits such as self improvement, emotional engagement, and control (Lupton and Tulloch, 2002). As such, humans readily show putative risk behaviour such as car driving, travelling by airplane, climbing mountains, boarding roller coasters, investing in stock shares, gambling in casinos, purchasing lottery tickets, smoking cigarettes, eating fugu fish, etc. Each of such activities is associated with its own inherent risk, either high or low. The actual risks can vary by many orders of magnitude and importance, some risks existing only theoretically while other risks may be a real threat to human health or even life (Faustman and Omenn, 2008). Benefit–risk analysis is the comparison of the risk of a situation to its related benefits and comprises a constellation of methods. The central question addressed within a ‘‘benefit–risk trade-off’’ framework is whether – and to which extent – a certain level of risk is acceptable. The methodological approaches and measurement methods to operationalize these benefit–risk tradeoffs are drawn from many scientific areas.
Food and nutrition are essential for life. The benefit of foods is, first and foremost, to provide nutrition (Bowman and Russell, 2006). Additional potential health benefits are associated with nutrition and health claims and are currently managed under EU Regulation 1924/2006 (Verhagen et al., 2010). Food contains many ingredients: macronutrients (fat, carbohydrates, protein, fibre, water), micronutrients (vitamins, minerals, trace elements) and non-nutrients (Bowman and Russell, 2006). Non-nutrients are either contaminants (pesticides, mycotoxins, heavy metals, microorganisms, etc.) (Kotsonis and Burdock, 2008) or other substances, some of which are claimed to have beneficial effects (carotenoids, flavonoids, terpenoids, glucosinolates, etc.) (Tapsell et al., 2006). Food is assumed to be safe and many laws are in place to secure the safety of food (EU 2000 White Paper on Food Safety; http:// ec.europa.eu/food/food/intro/white_paper_en.htm; http://ec.europa. eu/food/food/foodlaw/index_en.htm).
Thus, on the one hand, food contains necessary and beneficial ingredients, whereas, on the other hand, it may also contain potentially adverse ingredients. Moreover, food and food ingredients may be both beneficial and adverse. The beneficial and adverse potential may even be in the same food ingredient. For example, vitamins and minerals are necessary micronutrients for which recommended intake levels (RDA; recommended dietary allowances) have been set. However, too high levels of intake could result in adverse effects, and to this end maximum safe levels (tolerable upper intake levels) are determined (EFSA: http:// www.efsa.europa.eu/en/scdocs/oldsc/ndaintakevitaminsminerals. htm). There are many more examples: (fatty) fish reduces the risk of heart disease but can contain contaminants, fruits and vegetables are good for general health but they may contain pesticides, phytosterols lower blood cholesterol but also lower blood betacarotene levels and are potentially atherogenic; food heating kills microorganisms but it may produce acrylamide, etc. (www.brafo. org; Boobis et al., 2007).
Benefit–risk analysis is a new aspect in the area of food and nutrition. It envisages expressing both risks and benefits of foods and food ingredients into one currency, thereby allowing for a qualitative and quantitative comparison of adverse and beneficial effects. A benefit–risk assessment may help policy-makers to make more updated, informed, and balanced benefit–risk management decisions. In this context, it is important to realise that aiming to achieve maximal safety and application of a precautionary principle may be suboptimal from a public health perspective. The approaches and policies followed and measures taken to guarantee food safety may lead to too low levels or absence of ingredients from the perspective of benefits. In this, both the benefit–risk assessor and the benefit–risk manager share responsibility. The former should inform the latter, the latter should make evidenceinformed policy decisions. The benefit–risk manager needs to weigh the benefits and risks in a balanced way. In this, not allowing food benefits to occur in order to guarantee food safety is a risk management decision equally well as accepting some risk in order to achieve more benefits. In simple language: ‘‘any choice is a choice’’ or ‘‘doing nothing is equally well a choice as doing something’’. In addition to some scattered national initiatives, in Europe there are currently several major initiatives ongoing to explore the area of benefit–risk analysis for foods and nutrition. All of these projects are developing more or less similar methods and approaches to qualitatively and quantitatively compare risks and benefits, and to come to the ultimate decision in a tiered approach (Hoekstra et al., 2010). These projects are shown in Table 1.
In order to improve the scientific developments in benefit–risk assessment for food and nutrition, the SAFEFOODERA call on risk benefit analysis (http://www.safefoodera.net/text.cfm?ID=0-0- 135) requested to approach benefit–risk analysis for foods and nutrition from another (any other) perspective. In particular, it was requested to identify issues like economic and social aspects, case studies, etc. Whereas benefit–risk analysis is new in the food and nutrition area, there is already established experience with benefit–risk analysis in other areas, such as medicines, for which side effects are accepted (EMEA 2008: http://www.emea.europa. eu/pdfs/human/brmethods/1540407enfin.pdf). The experience with benefit–risk analysis in other scientific areas has been the starting point for the BEPRARIBEAN project (BEst PRActices in RIsk–BEnefit ANalysis). This project focuses on benefit–risk analysis, combining the aspects benefit–risk assessment, benefit–risk management and benefit–risk communication, which are the comparators to the classical and well-accepted risk analysis paradigm (Smith, 2002).
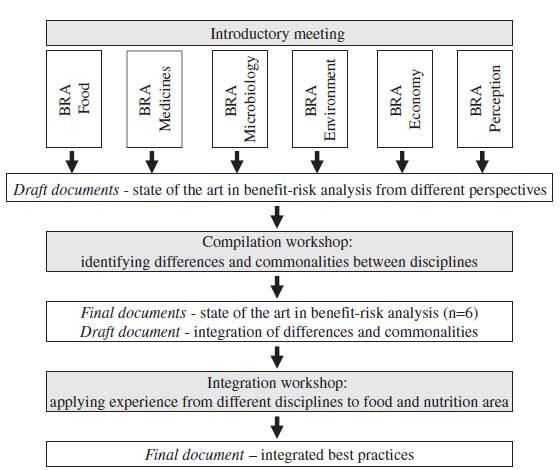
Therefore, the BEPRARIBEAN project approaches benefit–risk analysis for foods and nutrition from other perspectives, rather than duplicating existing activities, in order to push forth the area of benefit–risk analysis for foods. It is envisaged that benefit–risk analysis for food and nutrition can learn from experiences in other areas as well as vice versa. The project works from the benefit–risk experience in other areas. The structure of the project is simple and straightforward (Fig. 1): six teams of scientists prepared a state-ofthe- art paper in their respective scientific fields. Thereafter, the other partners contributed to the evolution and writing of the individual papers. On the basis of the experiences gained in the six state-of-the-art papers a final integration document will be prepared focusing on differences and commonalities in benefit–risk assessment. The project will be accompanied by meetings of participants as well as additional participants coming from sponsors and interested scientists. The project started in spring 2009 and will finish with an end conference in spring 2011. The final integration paper is envisaged to be published in the second half of 2011. The six state-of-the-art papers are published in this volume (Table 2). Finally the project has a website for further information: http://en.opasnet.org/w/Bepraribean.
We hope, that the outcomes of the BEPRARIBEAN project are providing crucial insights and are advancing the scientific developments in benefit–risk analysis in food and nutrition by learning from experiences in other areas.
| Acronym | Short project description | Website/reference |
|---|---|---|
| BENERIS | EU project ‘‘Benefit–Risk Assessment for Food’’ | http://en.opasnet.org/w/Beneris |
| BEPRARIBEAN | BEst PRActices in BEnefit–RIsk ANalysis | http://en.opasnet.org/w/Bepraribean |
| BRAFO | EU project ‘‘Risk Benefit Analysis of Foods’’ | http://www.ilsi.org/Europe/Pages/BRAFO.aspx |
| EFSA | EFSA working group on Human Health Risk–Benefit Assessment of Foods | http://www.efsa.europa.eu/en/scdocs/doc/1673.pdf
http://www.efsa.europa.eu/en/colloquiareports/colloquiariskbenefit.htm http://www.efsa.europa.eu/en/sc/scwgs.htm |
| PLANTLIBRA | PLANT food supplements: Levels of Intake, Benefit and Risk Assessment | http://www.plantlibra.eu/web/ |
| QALIBRA | EU project ‘‘Quality of life – integrated benefit and risk analysis’’ | http://www.qalibra.eu/ |
| State of the art in benefit–risk analysis for: | Coordinated by (institute and country) | Reference |
|---|---|---|
| Food and nutrition | National Institute for Public Health and the Environment (RIVM) and Maastricht University, The Netherlands | Tijhuis et al. (this volume) |
| Medicines | University of Ulster, United Kingdom | Luteijn et al. (this volume) |
| Food Microbiology | Matís, Iceland | Magnússon et al. (this volume) |
| Environmental Health | National Institute for Health and Welfare (THL), Finland | Pohjola et al. (this volume) |
| Economics and Marketing-Finance | Maastricht University, The Netherlands | Kalogeras et al. (this volume) |
| Consumer Perception | Nofima, Norway | Ueland et al. (this volume) |
Conflict of Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The preparation of this manuscript was funded through Safefoodera Project BEPRARIBEAN (Project ID 08192) by the Dutch Food and Consumer Product Safety Authority (VWA), the Research Council of Norway (RCN) and the Nordic Council of Ministers (NCM) and supported by Matís, The National Institute for Health and Welfare (THL), the University of Ulster and the National Institute for Public Health and the Environment (RIVM).
References
Boobis, A., Contor, L., Kind, P., Müller, D., Rechkemmer, G., Verhagen, H., Szoradi, A., 2007. BRAFO – risk–benefit analysis of foods. Ann. Nutr. Metab. 51 (Suppl. 1), 393 (P815).
Bowman, B.A., Russell, R.M., 2006. Present Knowledge in Nutrition, 9th ed. International Life Sciences Institute, Washington, DC.
Faustman, E.M., Omenn, G.S., 2008. Risk assessment. In: Klaassen, C.D. (Ed.), Casarett and Doull’s Toxicology. The basis Science of Poisons, 7th ed. McGraw– Hill, Medical Publishing Division, New York.
Hoekstra, J., Hart, A., Boobis, A., Claupein, E., Cockburn, A., Hunt, A., Knudsen, I., Richardson, D., Schilter, B., Schutte, K., Torgerson, P.R., Verhagen, H., Watzl, B., Chiodini, A., 2010. BRAFO tiered approach for benefit–risk assessment of foods. Food Chem. Toxicol. (epub ahead of print). <http://www.ncbi.nlm.nih.gov/pubmed/20546818>.
Kotsonis, F.N., Burdock, G.A., 2008. Food toxicology. In: Klaassen, C.D. (Ed.), Casarett and Doull’s Toxicology. The basis Science of Poisons, 7th ed. McGraw–Hill, Medical Publishing Division, New York.
Lupton, D., Tulloch, J., 2002. ‘Life would be pretty dull without risk’: voluntary risktaking and its pleasures. Health Risk Soc. 4, 113–124.
Smith, M., 2002. Food Safety in Europe (FOSIE): risk assessment of chemicals in food and diet: overall introduction. Food Chem. Toxicol. 40, 141–144.
Tapsell, L.C., Hemphill, I., Cobiac, L., Patch, C.S., Sullivan, D.R., Fenech, M., Roodenrys, S., Keogh, J.B., Clifton, P.M., Williams, P.G., Fazio, V.A., Inge, K.E., 2006. Health benefits of herbs and spices: the past, the present, the future. Med. J. Aust. 185, S4–24.
Verhagen, H., Vos, E., Francl, S., Heinonen, M., van Loveren, H., 2010. Status of nutrition and health claims in Europe. Arch. Biochem. Biophys. 510, 6–15.