EFSA: Guidance on human health risk-benefit assessment of foods
This page is a nugget.
The page identifier is Op_en4680 | |
|---|---|
| Moderator:Pauli (see all) | |
|
| |
| Upload data
|
Unlike most other pages in Opasnet, the nuggets have predetermined authors, and you cannot freely edit the contents. Note! If you want to protect the nugget you've created from unauthorized editing click here |
SCIENTIFIC OPINION
Guidance on human health risk-benefit assessment of foods
EFSA Scientific Committee
European Food Safety Authority (EFSA), Parma, Italy[1]
Contents
Abstract
The Scientific Committee of the European Food Safety Authority (EFSA) developed guidance for performing risk-benefit assessments of food. The document focuses on human health risks and human health benefits, and does not address social, economic and other considerations such as “cost-effectiveness” considerations. It is considered as essential that formulation of the problem precedes the risk-benefit assessment as such. Agreement between the risk-benefit assessor and the risk-benefit manager on the terms of reference should be reached in order to ensure that the outcome of the assessment is useful and relevant for the risk-benefit manager goals. A stepwise approach is recommended for the risk-benefit assessment, i.e. i) initial assessment, addressing the question whether the health risks clearly outweigh the health benefits or vice versa, ii) refined assessment, aiming at providing semi-quantitative or quantitative estimates of risks and benefits at relevant exposure by using common metrics, and iii) comparison of risks and benefits using a composite metric such as DALYs or QALYs to express the outcome of the risk-benefit assessment as a single net health impact value. The outcome of each step of the assessment should also include a narrative of the strengths and weaknesses of the evidence base and its associated uncertainties. After each step of the risk-benefit assessment, discussion should take place between the risk-benefit assessor and the risk-benefit manager on whether sufficient information has been provided or whether the terms of reference should be refined in order to proceed with the next step of the assessment. Two examples (selenium as an indispensable nutrient, and fish consumption) illustrate the proposed approach for risk-benefit assessment.
Summary
The European Food Safety Authority (EFSA) asked its Scientific Committee to prepare a guidance document for performing risk-benefit assessments of food related to human health risks and human health benefits.
Risk-benefit assessments are performed in different disciplines, under various perspectives and use a wide range of quantitative or semi-quantitative tools. In this opinion, guidance for performing riskbenefit assessments of food focuses on human health risks and human health benefits and does not address social, economic and other considerations such as “cost-effectiveness” considerations. The Scientific Committee notes that there is less experience with benefit assessment than with risk assessment and therefore proposes to mirror the risk assessment paradigm by introducing four steps for the benefit assessment, i.e. positive health effect identification, positive health effect characterisation (dose response assessment), exposure assessment and benefit characterisation. Following this approach will facilitate a transparent comparison of risks and benefits in the riskbenefit assessment.
Problem formulation should precede the risk-benefit assessment. Agreement on the terms of reference between the risk-benefit assessor and the risk-benefit manager is critical for ensuring a useful and relevant outcome for the risk-benefit manager goals.
After problem formulation, a stepwise approach is recommended using three steps: i) initial assessment, addressing the question whether the health risks far outweigh the health benefits or vice versa, ii) refined assessment, aiming at providing semi-quantitative or quantitative estimates of risks and benefits at relevant exposure by using common metrics, and iii) comparison of risks and benefits using a composite metric such as DALYs or QALYs to express the outcome of the risk-benefit assessment as a single net health impact value. At each of the three steps, both risk assessment and benefit assessment are usually performed at the population level. Where differences in the sensitivity to the agent under consideration exist or are assumed to exist in specific subpopulations, separate consideration of these subpopulations is needed.
After each step of the risk-benefit assessment, discussion should take place between the risk-benefit assessor and the risk-benefit manager on whether sufficient information has been provided or whether the terms of reference should be refined in order to proceed with the next step of the assessment. The outcome of each step of the assessment should also include a narrative of the strengths and weaknesses of the evidence base and its associated uncertainties. The overall magnitude of uncertainty associated with a risk-benefit assessment may often be large. This should not be regarded as implying a failure of the assessment; on the contrary, it provides essential information for decision-making and helps in identification of data needs.
A number of metrics which can be used in the risk-benefit assessment are described in the document. It should be noted that more than one metric will be needed to capture all dimensions of health for a risk-benefit assessment. It is important that the risk-benefit manager is aware of the limitations of the metrics used for measuring risks and benefits.
The Scientific Committee recommends that metrics used in risk-benefit assessment and weight factors associated to most common diseases should be internationally agreed upon in order to ensure harmonisation and recognition of the assessments.
The Scientific Committee recommends a close collaboration between risk assessors and benefit assessors in order to ensure that data generated by one or the other can be used in a broader riskbenefit assessment context. Furthermore, the development of hard biomarkers of effect for both risk and benefit is also needed.
Two examples of the approach for risk-benefit assessment are given. The first one (selenium, an indispensable nutrient) illustrates the case where the risk and the benefit are associated with one single agent, while in the second example (fish), the risk is due to one selected contaminant in food (methylmercury), whilst the benefit is due to other food components. The examples highlight the complexity of risk-benefit assessment, already when entering the first steps of the assessment.
Background as provided by EFSA
Where a food or food substance is recognised to have the potential to exert both health benefits and health risks it is important for risk-benefit managers to be able to weigh the risks against the benefits on the basis of a qualitative or quantitative risk-benefit assessment. However, there is currently no agreement on general principles or approaches for conducting a risk-benefit analysis for food and the assessment of risk to human health of food substances or nutrients is usually conducted independently of possible health benefits.
EFSA organised a scientific colloquium on risk-benefit analysis of foods in July 2006 to have an open scientific debate on the methods and approaches for risk-benefit analysis of foods4. There was a general consensus that a risk-benefit analysis should mirror the approach already agreed upon in the risk analysis, namely consist of a risk-benefit assessment part, a risk-benefit management part, and a riskbenefit communication part. The risk-benefit assessment should be comprised of 3 elements, i.e. risk assessment, benefit assessment and risk-benefit comparison. As for the risk assessment paradigm which is well established, the benefit assessment should also include the following steps: positive health effect identification, positive health effect characterisation (dose-response assessment), exposure assessment, and benefit characterisation. Finally the risk-benefit comparison should contain a means, quantitative if possible, to compare/weigh the potential human health risks against the potential human health benefits. For this a common scale of measurement (“composite metric”) for the risk and the benefit would facilitate the communication of the results.
It is considered that the decision to initiate a risk-benefit analysis would best be made on a case-by-case basis and, given the resources required to carry out such an analysis, should only be undertaken when clearly needed. Therefore the formulation of the problem (“why is the risk-benefit analysis being done, why do we need it?”) is pivotal; furthermore, it is emphasised that the question asked by the risk-benefit manager to the risk-benefit assessor should be clearly understandable.
Regarding tools/data available or needed to quantify the human health risks and health benefits it is considered that tools for classification of risks and of benefits would need to be developed, together with tools for comparison and prioritisation of risks and benefits. Both tools and data should be available, together with a common scale of measurement for risk and benefit. In order to provide confidence in the outcome of a risk-benefit assessment, the assumptions made for the assessment as well as the uncertainties embedded in the outcome should be stated explicitly.
It has been proposed at the EFSA Scientific Colloquium4 that the “state-of-the-art” of risk-benefit assessment had advanced beyond the brainstorming stage and that it was now time to advance to the ”learning by doing” stage. Although it may be premature at present to develop a prescriptive framework for risk-benefit assessment, it is suggested that a guidance document should be developed with respect to methodology, approaches, tools and potential limitations in the risk-benefit assessment.
Terms of reference as provided by EFSA
EFSA requests the Scientific Committee to prepare a guidance document for performing risk-benefit assessments of food related to human health risks and human health benefits. To this end the document should give considerations to the following issues:
- Scope and objective of risk-benefit assessment;
- Identification of situations for which a risk-benefit assessment might be appropriate;
- Guidance on problem formulation particularly considering the type of risk-benefit analysis needed;
- Development of a harmonised language to express risk and benefits;
- Usefulness of currently available toxicological, epidemiological and nutritional data to assess risk-benefit;
- Consideration of methods and approaches needed to assess the risks and benefits and to compare them, e.g. common scale of measurement for the comparison of human health risks and health benefits;
- Considerations on how animal and other data can be extrapolated to the human situation in order to facilitate human risk-benefit comparison;
- Identification of potential limitations of any risk-benefit assessment;
- Ongoing research activities, such as DG RTD projects and activities undertaken by other organisations in order to join efforts and aim at harmonised approaches for risk-benefit assessment;
- Recommendations on future initiatives to overcome current limitations.
Assessment
Introduction
In July 2006, EFSA organised a scientific colloquium on risk-benefit analysis of foods, during which it was proposed that the “state-of-the-art” of risk-benefit assessment had advanced beyond the brainstorming stage (van Kreijl et al., 2004) and that it was now time to advance to the “learning by doing” stage. Although it may be premature to develop a prescriptive framework for risk-benefit assessment, it was suggested that a guidance document should be developed with respect to methodology, approaches, tools and potential limitations in the risk-benefit assessment. Since then, several activities such as the Beneris, Qalibra, Brafo and Bepraribean projects, which EFSA has been following closely, have been commenced to address the issue of risk-benefit assessment. In February 2010, the EFSA Scientific Committee endorsed the draft guidance on “human health riskbenefit assessment of foods” for a 6-week public consultation. The 280 comments received from 19 interested parties were considered in April 2010 for finalising the present guidance document (EFSA, 2010).
Risk-benefit assessments are performed in different disciplines, under various perspectives (government, industry, patients) and using a wide range of quantitative and semi-quantitative tools. Examples are human medicine (e.g. assessment of the benefits and risks in the context of a new drug application) and engineering. Many of such assessments include socioeconomic considerations or aspects beyond human health that are not directly comparable and require value judgments to be compared.
The classic case where value judgments are needed is when the risk or benefit assessment is used as the basis of a cost-benefit analysis. In this case risks and benefits are given monetary values reflecting market prices directly or indirectly. The use of economic methods such as willingness to pay studies or co-joint analyses could be helpful in eliciting information on the consumer or citizen preferences and valuing the benefits and risks. The direct and indirect monetary costs of years of life lost through, and years of life spent with diet-related diseases, like cardiovascular diseases or cancer can be calculated based on morbidity and mortality statistics. The EFSA’s Scientific Committee, given EFSA’s remit, excluded social, economic and other considerations such as “cost-effectiveness” from its considerations. In this opinion, guidance for performing risk-benefit assessments of food related to human health risks and human health benefits is provided. The result of such an assessment will enable risk-benefit managers to take decisions and to formulate a strategy, taking into account other considerations such as social, economic or “cost-effectiveness” aspects.
Risk assessment - Definition
Risk assessment is "a process intended to calculate or estimate the risk to a given (sub)population, including the identification of attendant uncertainties, relating to exposure to a particular agent, taking into account the inherent characteristics of the agent of concern as well as the characteristics of the specific target system" (IPCS, 2004). For the purpose of this opinion the agent will be a food itself or a constituent of a food (incl. contaminants, microbes), and the target system is the human body.
Different organisations use different definitions of risk, depending on the focus of their activities. In the context of this opinion, the following definition of risk will be used:
Risk: The probability of an adverse effect in an organism, system9, or (sub)population in reaction to exposure to an agent (IPCS, 2004).
The terms hazard and adverse health effect have been defined for the use in risk assessment:
Hazard: Inherent property of an agent or situation having the potential to cause adverse effects on health when an organism, system, or (sub)population is exposed to that agent (slightly modified from IPCS, 2004).
Adverse (health) effect: a change in morphology, physiology, growth, development, reproduction or life span of an organism, system9 or (sub)population that results in an impairment of functional capacity, an impairment of the capacity to compensate for additional stress, or an increase in susceptibility to other influences (IPCS, 2004; FAO/WHO, 2006).
Notably hazard describes the exposure dependent potential of an agent to cause harm which in this context consists of an adverse effect on health. Therefore, adverse health effects caused by an insufficient intake, e.g. of an indispensable (essential) nutrient are not attributable to a hazardous property of that nutrient; while adverse health effects caused by excessive intake are. Accordingly, the evaluation of a nutrient could be done as a risk-risk comparison, by comparing the risk of inadequacy (deficiency or absence of a beneficial effect) to the risk of excessive intake (toxicity) (Renwick et al., 2004, EFSA, 2006a).
Benefit assessment - Definition
In common language a benefit provides an advantage, a help or an aid and beneficial is something which is helpful or good for something or someone. This means that risk and the term benefit in its conventional sense would not be a pair of corresponding opposite terms, while adverse health effect and positive effect on health are. There is also no term for the inherent potential of an agent (food) to cause beneficial effects on health which would correspond to the term hazard as applied in risk assessment.
In the context of this opinion and in line with the definition of risk, benefit is considered to consist of the probability of a positive effect on health (see box on “Benefit”). The reduction of a risk will also be considered as a benefit. Consequently, the following definition of benefit is used:
Benefit: The probability of a positive health effect and/or the probability of a reduction of an adverse health effect in an organism, system, or (sub)population, in reaction to exposure to an agent.
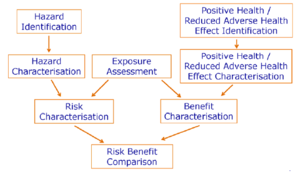
In contrast to risk assessment there is not much guidance published on how to perform benefit assessment of foods and food constituents. It is proposed in this opinion that positive health effects of nutrients, foods or constituents of food are assessed in a similar way to hazards, that is potential benefits should be identified, described, weighed and arranged according to their magnitude, and their doseresponse relationship should be characterised (see right side of Figure 1). Several concepts for assessing evidence for beneficial effects have been developed (Aggett et al., 2005; WHO/FAO, 2003; WCRF/AICR, 2007).
Risk-benefit assessment - Definition
The EFSA scientific colloquium on Risk-Benefit Analysis of Food (EFSA, 2006b) concluded that a risk-benefit analysis should mirror the approach agreed upon for risk analysis (IPCS, 2004; Codex Alimentarius Commission, 2005; FAO/WHO, 2006), and therefore should include a risk-benefit assessment, a risk-benefit management and a risk-benefit communication part. This opinion focuses on the risk-benefit assessment.
In the risk-benefit assessment, the probability of an adverse health effect or harm (both incidence and severity) as a consequence of exposure can be weighed against the probability of benefit, if both are known to be possible.
The Scientific Committee proposes the following terms and their counterparts for the assessment of the probability of harm (= risk) and of the assessment of the probability of the positive health effects (= benefit).
| Risk assessment | Benefit assessment |
|---|---|
| Hazard identification | Positive health effect/reduced adverse effect identification |
| Hazard characterisation (dose response assessment) | Positive health effect/reduced adverse effect characterisation (dose response assessment) |
| Exposure assessment | Exposure assessment |
| Risk characterisation | Benefit characterisation |
Figure 1 illustrates the proposed procedure for a risk-benefit assessment which consists of two separate and independent arms of assessing the risk and the benefit, respectively. Both assessments include four steps on both sides that are comparable: identification of the possible hazards and positive / reduced adverse health effects together with their biological mechanisms if possible; characterisation of the identified hazards and positive / reduced adverse health effects with respect to severity, reversibility and dose-response relationship; and characterisation of the risk and the benefit, that is the probability of each identified hazard or positive health effect to occur in a population or population group. The exposure assessment is positioned as a central part of the risk-benefit assessment and should take into account all relevant dietary and non-dietary sources. Finally, the risk-benefit comparison will weigh the risks against the benefits.
Different scenarios for the risk-benefit assessment can be foreseen due to the nature of the benefits and
risks. The different scenarios for different risks and benefits connected with one food and concerning
the same or different populations are outlined in Section 2.1.
Proposed approach for risk-benefit assessment
The guidance is primarily designed for the need of the EFSA Scientific Panels and Committee, as well as for Member States’ Competent Authorities who have to provide scientific advice to risk-benefit managers. However, the stepwise approach proposed in this section is also useful for other parties, e.g. academia or industry.
Examples of situations for which a risk-benefit assessment might be appropriate
Risk-benefit assessment would be appropriate in situations, such as, but not restricted to:
- Where a single compound or food constituent has both positive and negative health effects. These effects may occur: i) in the same population, e.g. for zinc, vitamin A, phytosterols, iron; ii) in different populations e.g. for folic acid fortified food, where the prevention of neural tube defects in the unborn child should be compared with potential hazards, such as masking of vitamin B12 deficiency in the elderly, dementia or colon cancer.
- Where similar levels of dietary exposures can be associated with both risk and benefit.
- Where positive and negative health effects, either in the same or different populations result from different components in the same food e.g.: i) fatty fish, where the main potential beneficial effects related to prevention of cardiovascular diseases by n3 fatty acids need to be compared with the potential negative health effects of environmental pollutants such as dioxins or PCBs, or ii) consumption of vegetables, where the positive effects such as supplying of micronutrients and prevention of certain types of cancer should be weighed against the potential hazards of the presence of nitrates, such as methaemoglobinaemia in infants and formation of carcinogenic nitrosamines. Before the start of an intervention, such as folic acid fortification, or fluoridation of drinking water.
- Where a significant change of dietary consumption patterns has occurred or may occur in the future, e.g. substituting sugar by low-calorie sweeteners.
- Where chemicals are used to reduce microbial contamination, e.g. use of disinfection processes.
- Where the beneficial effect, such as enhanced retention of nutritional value resulting from improved processing procedures, requires to be assessed against the negative effects associated with a greater survival of foodborne pathogens.
- Where new knowledge emerges with major implications for either the risk(s) or the benefit(s) in a previous risk assessment, benefit assessment or risk-benefit assessment. For example the possible association between folic acid consumption and colon cancer.
It is to be noted that risk-benefit assessment does not replace procedures required by existing European legislation, e.g. safety assessment and authorisation of a food additive.
Problem formulation
Problem formulation should precede the risk-benefit assessment because a clear formulation of the problem is critical for ensuring a useful and relevant outcome of the risk-benefit assessment. Problem formulation in risk assessment was addressed among others by US EPA (1998) and by the FOSIE project (Food Safety in Europe: Risk assessment of chemicals in food and diet (Renwick et al., 2003)). In contrast, problem formulation in benefit assessment or risk-benefit assessment has received much less attention to date.
Problem formulation is the responsibility of the risk-benefit manager and preferably should be conducted in dialogue with the risk-benefit assessor to ensure that the outcome, i.e. the formulated Terms of Reference, is appropriate for the risk-benefit management goals. The Terms of Reference should define the risk-benefit question to be addressed. Risk-benefit questions are of two main types:
- What is the balance of risks and benefits caused in a population by a particular diet (often the current diet) or dietary component (e.g. fish)?
- What would be the net health impact of a specified change in the diet, e.g. a public health intervention, a new product, or a change in consumer preferences – Comparison of alternative scenario(s) to a reference (current) scenario.
The Terms of Reference should specify which type of risk-benefit question is asked and the diet, dietary element or dietary change to be assessed. It will generally be important also to specify the population to be considered, e.g. the whole European population, one or more national populations, or a particular subpopulation (e.g. children, immunocompromised, etc.), as this may be important for the risk-benefit manager and can have significant time and/or data implications for the assessment.
The Terms of Reference should also specify the timetable for completing the assessment, and optionally it may specify whether and which stakeholders should be involved in the process. In some cases the Terms of Reference may identify some types of health effects that should be included in the assessment, if they are of particular interest to the risk-benefit manager or to stakeholders. However, this is not essential, because identifying relevant potential effects is an intrinsic part of the risk-benefit assessment.
Proposed approach for risk-benefit assessment
As mentioned in Chapter 1 the risk-benefit assessment should comprise three elements: risk characterisation, benefit characterisation and a comparison of risks and benefits. As shown in figure 1, this implies that both hazards and positive health effects need to be characterised, and that by taking the results of the exposure assessment into consideration, risks and benefits are characterised. The final part of the risk-benefit assessment comprises a direct comparison of potential health risks and potential health benefits. One of the conclusions of the EFSA scientific colloquium on Risk-Benefit Analysis of Food (EFSA, 2006b) was to follow a stepwise approach.
After problem formulation, the Scientific Committee recommends a stepwise approach for the riskbenefit assessment using the following steps:
- Step 1, Initial assessment
- Step 2, Refined assessment
- Step 3, Assessment using a composite metric (see section 2.4)
The Scientific Committee underlines that after completion of each step by the risk-benefit assessor, discussion should take place with the risk-benefit manager on whether sufficient information has been provided and the assessment can stop. If this is not the case, new Terms of Reference need to be agreed upon in order to proceed with the next step.
While the risk-benefit assessor is responsible for stating the level of evidence available from the data for both risks and benefits, the risk-benefit manager concludes whether the level is adequate to come to decisions. In general, for risk assessment, it is appropriate to take a conservative approach in order to protect public health. However, for benefit, the manager frequently requires the evidence to be convincing.
For all the steps in the risk-benefit assessment, the rationale for following a certain approach and for selecting specific parameters should be clearly described. The risk-benefit assessment should include a description of the assumptions and uncertainties, and explain the outcome. This will help the riskbenefit manager to understand its relevance in relation to the management decisions to be taken.
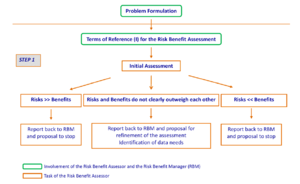
Step 1 – Initial assessment
This step (Figure 2) addresses the question of whether the health risks far outweigh the health benefits or vice versa. In order to do so, risks and benefits are considered separately and their health impacts are compared to conclude whether the risks clearly outweigh the benefits or the benefits clearly outweigh the risks. To make such a comparison, all relevant factors related either to a potential health risk or to a potential health benefit need to be considered.
Due to the inherent uncertainties in this assessment, this step can best be performed by addressing two different scenarios:
- Scenario 1: Estimate the risks at a high dietary exposure to the relevant agent(s) in food, together with the benefits at a low dietary exposure to the relevant agent(s) in food, i.e. upper bound for risks and lower bound for benefits. If by doing so, risks are still much smaller than benefits (risks << benefits), this ends the risk-benefits assessment, as the assessment will have to focus on benefits. In all other cases, a proposal will be made to the risk-benefit manager to refine the assessment by proceeding to step 2. For example, if with this scenario, it appears that the exposure of the population is clearly below an existing health based guidance value (such as ARfD, ADI, TDI, UL) for the compound(s) that needs to be considered, then there is no appreciable health risk. In that case, the question that needs to be answered is whether the available evidence is strong enough to conclude on whether there is a potential beneficial effect for the situation being evaluated. In the case of indispensable nutrients, if exposure is at or above dietary reference values or nutrient status parameters are within the normal range, there is no appreciable risk of nutrient insufficiency and the assessment can stop.
- Scenario 2: Estimate the risks at a low dietary exposure to the relevant agent(s) in food, together with the benefits at a high dietary exposure to the relevant agent(s) in food, i.e. lower bound for risks and upper bound for benefits. If by doing so, risks are still much greater than benefits (risks >> benefits), this ends the risk-benefit assessment, as the assessment will have to focus on risks. For example, when there is no evidence for a health benefit for a dispensable nutrient (e.g. exposure below effective dose), the remaining question is whether there is a possible health concern.
When there is either no appreciable health risk (based on scenario 1) or no appreciable health benefit (based on scenario 2), this is reported back to the risk-benefit manager with the proposal to stop the assessment.
In all other cases, a proposal will be made to the risk-benefit manager that the assessment of the risks and the benefits should be refined by either acquiring new data or proceeding to step 2. A dialogue should follow to agree on new Terms of Reference (II), taking into account:
- Endpoints and population(s) to be considered to adequately reflect the objectives of the riskbenefit assessment (see section 3.1).
- Possible refinement of the exposure assessment, e.g. by incorporating probabilistic exposure assessment (EFSA, 2006c) or specific exposure scenarios as indicated by the risk-benefit manager.
- Potential for quantification of hazards and positive heath effects, e.g. by dose response modelling (EFSA, 2009)
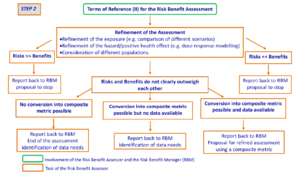
Step 2 – Refinement of the assessment
The approach taken in step 2 (Figure 3) will be determined by the Terms of Reference (II). The aim is to provide, depending on the available data, semi-quantitative or quantitative estimates of risks and benefits at relevant exposures, where possible using common metrics, i.e. a measurement expressed in the same unit, for example, incidence or mortality (see section 2.4).
Possible outcomes might be:
- Estimates of the proportion of the population, or a relevant subgroup with exposure that is above a health-based guidance value or below a dietary reference value or a minimum dose level for a positive health effect
- Estimates of disease incidence or mortality occurring at a particular exposure level, and the impact of changing the exposure, e.g. by dietary intervention such as fortification or advice
- Estimates of the proportion of the population (or subgroup) that could become ill based on a probabilistic approach to both exposure and susceptibility
- Probabilistic distribution of the health benefit and health risk in combination with a quantification of their inherent uncertainties.
In all cases the uncertainties in the estimations should be described, and quantified to the extent possible (see section 4).
Where risks do not markedly outweigh benefits (risks not << benefits), or vice versa (risks not >> benefits), there may still be evidence on the basis of one or more common metrics for a net risk or a net benefit. However, it is the decision of the risk-benefit manager as to whether this will suffice to support policy or whether additional refinement will be necessary. This could either be via step 3, or by acquiring new data.
Hence, the outcome of the risk-benefit assessment is reported back to the risk-benefit manager and a dialogue between the risk-benefit assessor and the risk-benefit manager should follow to agree whether or not to proceed to step 3 with composite metrics, i.e. a single measure that reflects a number of dimensions of health, including morbidity and mortality (see section 2.4). To assist in this decision, the outcome of step 2 should include an assessment of whether it would be possible to derive composite metrics, on the basis of available information. This will help the risk-benefit manager in deciding on whether conversion into a composite metric would be necessary and, if so, feasible. If necessary and feasible, this will require the formulation of new Terms of Reference (III).
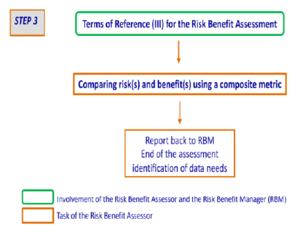
Step 3 – Comparison of risks and benefits using a composite metric
The Scientific Committee recommends that the Terms of Reference (III) should indicate whether there is a preference on which composite metric should be used to compare and/or aggregate the risks and benefits. In step 3 (Figure 4), composite metrics are used to combine two or more of the following elements: increases or decreases in morbidity, mortality, disease burden, and quality of life. The choice of composite metrics should be made on a case by case basis, based on the specific riskbenefit question, identified hazards and positive health effects. The choice of a composite metric should be justified.
The outcome of the risk-benefit assessment can be expressed as a single net health impact value. The Scientific Committee recommends however, when reporting to the risk-benefit manager on the outcome of the risk-benefit assessment, to provide as well the respective health impact values expressed in the selected composite metric for each relevant health effect and each relevant sub population with their respective uncertainties. The net outcome of the risk-benefit assessment should therefore not be considered in isolation. When reporting to the risk-benefit manager the risk-benefit assessor needs to consider that the result “is more than a number” and should be considered together with the outcome of the Step 2 assessment.
In some cases the outcome of the assessment might not lead to a clear conclusion because the inherent uncertainties are too large. In reporting back to the risk-benefit manager, recommendations on data needs to reduce uncertainty should be made.
Metrics used in risk-benefit assessment
Health effects can be assessed in a number of different dimensions, such as incidence of effect, severity of effect, morbidity and mortality rate, and in the case of positive health effects also quality of life. More than one metric will be needed to capture all dimensions of health for a risk-benefit assessment.
A common metric is a measurement expressing risks and benefits in the same unit, for example, incidence or mortality.
A composite metric for risks and benefits reflects a number of dimensions of health, such as severity of the disease, morbidity and mortality, expressed in the same unit.
The terminology that is used for the metrics of morbidity, mortality and disease burden varies. Therefore the Scientific Committee recommends that the definitions in the dictionary of epidemiology (latest edition, International Epidemiological Associations, Dictionary of Epidemiology, Editor Miquel Porta) be used. Alternatively, the terms used should be explicitly defined in each risk-benefit assessment.
Effects expressed in a common metric can be compared, but care must be exercised in the interpretation of the comparison. Comparing the incidence of a minor ailment with that of a major disability is obviously of limited value. Even comparison of the incidence of the same effect may be problematical due, for example, to differences in severity or age group affected. Whilst mortality metrics are more directly comparable, these also have limitations; they do not capture the total number of people affected such as when risks and benefits occur in different sub-populations varying in size. Similarly, mortality rate does not take into account the severity of the cases. Death may occur suddenly, or it may occur only after a prolonged period of ill health. Moreover, this metric, when expressed as mortality rate standardised for a given number of the population does not indicate whether the deaths are occurring in particular age groups, which may be an important consideration for risk-benefit managers.
Whilst composite metrics, such as disability or quality adjusted life years (DALYs or QALYs), can be used for direct comparison of effects, it is important to recognise that not all relevant dimensions are captured in these metrics, for example, whether the effect is in children or adults. This is because these metrics combine incidence with life years to obtain an estimate of years saved or lost respectively, so that a few young people with many years of potential life can give an equivalent value as a larger number of elderly people with far fewer years of potential life. In addition some of the DALY or QALY weightings are open for discussion.
There are some aspects of positive health effects that are difficult to quantify for inclusion in the DALYs or QALYs. Currently, generally agreed metrics for positive health effects and well being are lacking, in part because there are no agreed weighting factors for positive health effects. It is recommended that further work be undertaken to define metrics to measure positive health effects and well being.
It is important that the risk-benefit manager is aware of the limitations of the different metrics used for measuring risks and benefits. Metrics for assessing the risks and benefits are presented in Appendix A. The reader is referred to section 2.3 for a description of the stepwise approach proposed for the riskbenefit assessment.
Step 1 Initial assessment
In this case the question is whether the risks by far (>>) outweigh the benefits or vice versa. The risks and benefits should therefore be analysed separately.
No specific health metrics are used in this step. Rather, exposure is compared with agreed health based guidance values, such as ADI or TDI for risk and RDI or minimum dose levels for a positive health effect.
Step 2 Refinement of the assessment
In contrast to step 1 where risks and benefits were assessed at upper and lower bounds of exposure, in step 2, the risks and benefits are assessed semi- quantitatively or quantitatively at relevant exposures. For example, the number of people in whom dietary intake exceeds a health based guidance value could be estimated.
The metrics to be used for this step should include estimates of morbidity (prevalence and/or incidence), and mortality, some of which will be common metrics. Metrics for disease burden can be particularly useful for capturing benefits, where these are a consequence of a reduction in disease risks. Where positive health effects are to be assessed, suitable metrics, when available, should be used for measuring the benefits.
Step 3 Comparison of risks and benefits using a composite metric
In this step, risks and benefits are compared using composite metrics such as DALYs or QALYs. The outcome of this step can be expressed as a single net health impact value, but must be interpreted with caution.
Specific aspects in risk-benefit assessment
Importance of the selected endpoint(s) and the subpopulation(s) considered in the assessment
The endpoint(s) proposed for assessment of risk or assessment of benefit should have biological relevance to the outcome of concern. Hence, for risk, the endpoint should represent an adverse effect. Likewise, for benefit, the endpoint should represent a desirable change in health status or a likely positive consequence for health or well being, for example resistance to infection. This requirement carries with it the implication that the endpoints selected for use in the assessments will ideally have consequences for, or reflect, morbidity or mortality. Often, however, there will be uncertainty about this relationship and hence use of this criterion for the selection of endpoints will not always be possible. The assessment should therefore include a narrative of the strengths and weaknesses of the evidence base (level of evidence), and the associated uncertainties.
For indispensable nutrients, the obvious benefit endpoint will be the reduction of risk for nutrient deficiency. It is conceivable that there may be additional endpoints for benefit associated with the substance, e.g. a reduced risk of some forms of cancer with vitamin D intake in excess of recognised nutritional requirements. However, there may also be an increased risk of adverse effects. Each of these endpoints will need to be characterised separately.
Although each of the three steps of the risk-benefit assessment can be performed at the population or the individual level, in a public health context, both risk assessment and benefit assessment are usually performed at the population level. Where differences in the sensitivity to the adverse or beneficial effects of an agent under consideration exist or are assumed to exist in specific subpopulations, separate consideration of these subpopulations is needed. An assessment of all endpoints in all subpopulations will not always be necessary; the assessment should focus on the situation where the endpoints have the greatest impact on health or where there is the greatest uncertainty. Risk-benefit assessment for an individual would require additional specific information, such as dietary habits or genetic characteristics.
Types of data
The confidence in the relationship between the exposure to an agent and consequences for human health will depend on the type of data. For example, for benefit data obtained in intervention studies in human volunteers, the relationship for human health can be very strong, whereas for data from studies in vitro the relationship is likely to be much weaker. Sources of information may be in silico, i.e. simulation and modelling, in vitro, in vivo in experimental animals, observational and interventional human studies. For several of these study types, guidance is available on study design and reporting, e.g. OECD test guidelines. Adherence to such guidance reduces uncertainty as to the reliability of the data, for example through external quality assurance and adherence to good laboratory practice, but does not necessarily ensure relevance. Hence, expert judgment will always be necessary in interpreting the significance of the results of a particular study with respect to either risk or benefit to human health.
The type of data for endpoints may be categorical, ordinal or continuous. Examples are, respectively, number of fatal myocardial infarctions, mild – moderate – severe liver damage, serum potassium concentration. Appropriate descriptive statistical methods should be used in summarising such data sets. Information should be provided on study design (e.g. species and strain, sex, route of exposure, vehicle, duration, age of animals), analytical methodology, performance characteristics, number of replicate determinations, historical control data. Also for data obtained in humans, details on study design should be provided, e.g. the characteristics of the study population, matching of any control group, possible confounding factors and power to detect an effect size of a given magnitude or incidence.
A number of endpoints have been proposed for the assessment of positive health effects, e.g. number of healthy life years and life expectancy, motor, cognitive, neurologic and metabolic function, wellbeing, satiety and hunger (Asp et al., 2003). As mentioned before, the methodology for quantifying such endpoints is less well developed than that for assessing adverse health effects. There is increasing interest in the use of biomarkers in assessing biological responses. It is anticipated that there will be considerable advances in this area in the coming years. As in other areas of science, the use of any biomarker should be accompanied by a full appreciation of its limitations, as well as its advantages. As indicated above, assessments should ideally be performed on endpoints of known adverse or beneficial effects on health, the so called hard biomarkers. Only rarely will a biomarker be sufficiently robust for this to be the case. In such instances, the biomarker would be considered a surrogate endpoint. The relevance and validity of biomarkers should be established before they can be used as surrogate endpoints to replace frank endpoints in risk or benefit assessment. On the other hand, biomarkers reflecting intermediate changes, which although necessary are not sufficient by themselves for a biological outcome, may still be of value in providing supportive information for the assessment.
Subpopulation selection
Risks and benefits may occur in the population at large. However, the benefit(s) may be greater in one subpopulation, whilst the risk(s) may be greater in a different subpopulation. Information on both subpopulations, those at risk and those at benefit, will be required to enable risk-benefit management decisions to be made on the basis of the most relevant information. If these subpopulations are not identifiable by pre-defined criteria, then specific assessments cannot be performed, e.g. in the case of a genetic polymorphism that is not routinely screened.
Use of human data for exposure and effect
Exposure █ PFS | in PFS | What's this?
The nature and quality of the dietary intake measurement is an important determinant of the adequacy
of the exposure data for both risk and benefit assessment. Different methods are available, which are
intended to measure the habitual food and/or supplement intake over a defined period of time. These
methods are in various degrees susceptible to confounding and different biases and need to be
carefully interpreted.
In many cases, food consumption surveys are conducted primarily for nutritional purposes. Although there are some limitations which have to be taken into account, these surveys can be used in risk-benefit assessment. Repeated 24-hour recall dietary surveys, food-frequency questionnaires, one- to seven-days diaries and duplicate diet studies provide increasingly more robust data on dietary intake but are also increasingly complex and resource intensive, whilst the subject compliance decreases with study complexity. The assessor should be aware of the differing reliability of the exposure data and of their origin.
Moreover, the quality of dietary intake data depends both on the reliability and on the natural variability of the composition data for foods. Not all (computerised) food composition databases provide information on the number of samples analysed, analytical methodology and distribution of analysed values. Levels of nutrients, residues and contaminants in foods are rarely measured parallel to the assessment of food consumption; mostly results from market basket investigations or regular monitoring activities are combined with available food consumption data.
In epidemiological studies, biomarkers may be used as a measure of exposure to an agent (e.g. blood levels, toenail concentrations, DNA adduct). Such biomarkers of exposure reflect the internal dose and exposure from all sources. When such biomarkers are used, back-calculation to dietary exposure is often needed, using kinetic modelling. In addition to model uncertainty, there can be uncertainty in identifying the contribution of a specific route of exposure (i.e. food) against other sources (e.g. inhalation).
Effects
Human data related to both adverse and positive health effects of substances in food reflect real-life exposures. Human studies can have either an experimental (e.g. clinical trials or intervention studies) or an observational (e.g. case control studies and cohort studies) design.
Intervention studies, ideally performed as randomised-controlled-trials (RCT) have the advantage of good control for confounders and biases when studying a cause-and-effect relationship between diets/dietary constituents and both adverse and positive health effects. Therefore they provide the highest strength of evidence. Due to ethical, financial and practical reasons, it is unlikely to be possible to conduct an experimental study in humans looking for adverse effects as the primary endpoint. Adverse health effects may be incidentally observed in studies conducted in the expectation for beneficial effect and should be systematically recorded and analysed. It should be noted that health outcomes with a long latency (e.g. cancer or heart disease) can not be adequately investigated in studies of short duration. Intervention studies can only be carried out once toxicological screening has given reasonable evidence that harm will not occur. Randomised, double-blind placebo-controlled intervention studies are best used to study beneficial outcomes of minor components of the diet such as trace elements or vitamins, as there will be no significant perturbation of the diet and compliance can be expected to be high. Because of the high costs of large-scale intervention studies, exposure is usually limited to a few or even one exposure level, thus limiting information regarding the exposure– response relationship, which is a major limitation.
Observational epidemiologic studies are based on dietary exposure that is more relevant to the general population. Observational studies cannot establish causality of a relationship based only on a statistical association. High-quality observational epidemiologic studies can, however provide strong arguments for causal associations for both risks and benefits and have less practical limitations if performed and evaluated according to strict quality criteria.
In summary, intervention trials (Randomised Controlled Trials) provide the strongest evidence for a causal relationship between risk/benefit and dietary exposure and have the lowest chance for potential bias to occur, whilst the influence of confounding on the results of observational studies can be reduced by appropriate design and data analysis.█ PFS | in PFS | What's this?
Considerations on how animal and other data can be extrapolated to the human situation in order to facilitate human risk-benefit comparison
Risk-benefit assessments may deal with microorganisms and/ or chemicals including nutrients. For each of these categories the assessment of risks and benefits is carried out independently and the type of data underlying the assessments may differ. Therefore, an important consideration to be taken into account when making risk-benefit assessments for the human situation is the nature of the data on which these assessments can be based.
For chemicals other than nutrients, data for the risk assessment mostly result from animal studies. It is generally assumed, in the absence of evidence to the contrary, that the effects occurring at lowest doses in animal studies will also be the most sensitive effects in humans. The extrapolation requires conversion of the dose-response data into a human equivalent by scaling, using for instance bodyweight or surface area or a more sophisticated method like physiologically based biokinetic (PBBK) or biodynamic (PBBD) modelling. It is important to stress however that such models and the data required to define them are generally not readily available. For chemicals other than nutrients, for which information on benefits exists, such information often comes from human epidemiology and/or intervention studies.
For nutrients and microorganisms, although dose-response data are often limited, data for risk and benefit assessment are mainly derived from human studies. Therefore, extrapolations from animal data to the human situation will not be necessary. In some cases of nutrients, only animal data are available and in such cases extrapolation from animals to humans will be required, e.g. tolerable upper intake level of molybdenum (EFSA, 2006a).
In the process of risk assessment these extrapolations from animals to humans are frequently made using uncertainty factors. This method applies to non-genotoxic compounds for which health-based guidance values like Acceptable Daily Intake (ADI), Tolerable Daily Intake (TDI), or Acute Reference Dose (ARfD) are derived by dividing the no-observed-adverse-effect level (NOAEL) or the Benchmark Dose Lower confidence limit (BMDL), identified in an animal toxicity experiment, by uncertainty factors, usually including a factor of 10 for inter-individual differences, and a factor of 10 for interspecies differences. The health-based guidance values, thus established, define exposure values at or below which no adverse effects in humans are expected.
If the health based guidance values are exceeded, the risk level should be estimated. For that, the doseresponse curve from the animal studies has to be converted to a human equivalent, by assuming that the dose response curves in animals and humans are parallel.
For compounds that are both genotoxic and carcinogenic, a quantitative risk assessment for the human situation requires the development of biologically relevant models for extrapolation of animal cancer data determined at high levels of exposure to cancer risks at realistic and much lower human levels of exposure. Currently, this low dose cancer risk extrapolation is known to be dependent on the statistical models applied, which are not biologically-based. The low dose risk estimates are known to vary by orders of magnitude with the extrapolation model applied (COC, 2004, EFSA 2005). Therefore, for assessing the risk of this type of substances, the margin of exposure (MOE) approach was introduced by EFSA (2005) as a harmonised approach for the assessment of substances that are both genotoxic and carcinogenic. The MOE approach is applicable in step 1 and 2 of the risk-benefit assessment. However the MOE is not a quantitative cancer risk estimate and therefore cannot be translated into a composite metric in step 3. Therefore, at present and until biologically-based methods for extrapolation from animal data are developed, the quantitative assessment of cancer risks within the framework of risk-benefit assessment has to be based on human epidemiological data. Even when using epidemiological data, if this is based on occupational exposure, it may not be possible to obtain reliable estimates of risk at the much lower levels of dietary exposure.
In the field of nutrition, no standard procedure has been defined to assess if positive health effects observed in animals can be reproduced in or are relevant for humans. Established dietary reference values for indispensable nutrients on the benefit assessment side may be considered as broadly equivalent to the health-based guidance values derived from the risk assessment.
Uncertainties in the risk-benefit assessment approach
Uncertainty has been described in the EFSA guidance document on uncertainties in dietary exposure assessment (EFSA 2006c) as resulting from limitations in scientific knowledge, and it can often be reduced by further investigation. Although aimed at exposure assessment, the guidance has also been used for uncertainties in toxicity (e.g. EFSA Panel on Contaminants in the Food Chain (CONTAM), 2009), and the approach is sufficiently general that it can be applied to the assessment of adverse and positive effects, and their net health impact after conversion into a composite metric.
The overall magnitude of uncertainty associated with a risk-benefit assessment may often be large. This should not be regarded as implying a failure of the assessment; on the contrary, it provides essential information for decision-making (Codex, 2010) and helps in identification of data needs. Uncertainty should be characterised at each step of the assessment, as described below.
Uncertainty in the hazard and the positive health effect characterisation
Identification of adverse and positive health effects involves a number of qualitative uncertainties with respect to limitations in knowledge on the full range of possible effects. For a substance that has been subject to comprehensive systematic toxicological evaluation, the major effects will be known, although there may be uncertainty about mode of action and human relevance of observations seen in experimental animals or in vitro models. For substances that have been less extensively investigated, it should be possible to identify the key data gaps as uncertainties. However in both these circumstances there may be additional uncertainty related to emerging scientific understanding, for example effects such as intolerance, behavioural changes, combined effects with other substances, positive health effects are not evaluated systematically in the same way as toxicological effects. The positive effect of an indispensable nutrient in correcting deficiency is well established, but only applies to individuals who are deficient. Other purported positive effects, such as improved well-being, may be claimed but not substantiated.
The relevant hazards and positive health effects may differ for different subgroups. In some circumstances it may be possible to identify specific subgroups with greater potential for risk or benefit, such as pregnant women when considering beneficial or adverse developmental effects. For other effects it may not be possible to identify the subgroup with greatest risk or benefit. A 10-fold uncertainty factor to allow for the unknown extent of the individual variability in toxicokinetics and toxicodynamics is commonly incorporated into health-based guidance values. So far, such factors have not been identified for beneficial effects. Nutrients are subject to physiological regulation (absorption, distribution, metabolism, storage) which may limit the range of inter-individual variability, and it will be necessary to describe uncertainty on a case-by-case basis. For microbial risks, it is assumed that the young, old and immuno-compromised are appreciably more susceptible than the healthy adults.
Information on doses with or without an effect will be needed for both the risk and the benefit assessments. In practice dose-response data are likely to be more fully characterised for chemicals subject to approval processes than for contaminants, microbial agents, or nutrients and other beneficial components of foods. The uncertainties in the hazard and the positive health effect characterisation will differ depending on whether the data are from animal studies, human populations or selected subgroups.
As discussed earlier, randomised controlled trials to investigate benefits generally do not define the dose-response relationship. For observational epidemiological studies, bias, confounding factors and limitations in exposure assessment result in uncertainty in characterising the dose-response relationship (see section 4.2).
Uncertainty in the exposure assessment
Most exposure assessments require information on food consumption and on the occurrence of the hazardous or beneficial agent in different foods. Often these data are derived from different sources, and specific information relating to relevant subgroups may be lacking. Different approaches are likely to be required for nutrients, non-nutritive chemicals and micro-organisms, because of changes during production, processing and cooking, or whether the risks/benefits relate to acute or chronic exposure. In the step-wise procedure, the first assessments are likely to rely on predictions based on generic exposure scenarios. In subsequent steps, additional data on occurrence and consumption will allow the exposure assessment to be refined.
Microorganisms multiply, survive or die along the food chain from farm, through the processing and retail, to fork. The dynamics of microbial growth and survival can be a source of uncertainty. Hence, the number of microorganisms at the point of consumption is variable and uncertainties will arise as a function of initial contamination and variability in conditions along the food chain (e.g., temperature, pH, salinity, water activity).
Uncertainty in risk-benefit comparison
In step 1 of a risk-benefit assessment, a narrative description of risks and benefits at different levels of exposure, with a systematic evaluation of the associated uncertainties may provide an adequate basis for risk-benefit managers to make decisions. If step 2 involves modelling of the dose response relationships for health effects and/or a probabilistic exposure assessment, it should be possible to quantify the statistical uncertainty, for example by calculation of confidence intervals. However, it should be made clear that this does not capture all of the uncertainty and there is still a need for a description of the underlying assumptions and different types of uncertainty in the separate assessments, such as the human relevance of data derived from animal experiments and the limitations of epidemiological studies. Further consideration of uncertainty is likely to be required if the riskbenefit assessment proceeds to step 3 using a composite metric approach. It will then be important to be transparent about the underlying assumptions used in converting to the composite metric. For example, value judgements are inherent in the weight factors for disease severity used for calculating DALYs and QALYs and assumptions made about survival times for different health endpoints may vary for different countries and regions.
Examples of risk-benefit assessment
This section provides two examples to illustrate the types of issues that need to be considered in conducting a risk-benefit assessment. These include identification of the benefits, the risks and the relevant subpopulation(s). It is important to identify early in problem formulation whether risks and benefits are likely to occur in the same or different subpopulations. Some consideration of the type of assessment that will be feasible is provided, based on the type and extent of information available. For example, data may come from human trials, experimental animals, or epidemiological observations. The extent of data may be such that only information following a single dose level is available (e.g. in many clinical trials), the evidence of benefit may be very equivocal, etc. The examples have been chosen to represent two different scenarios, within the framework of the approach to risk-benefit assessment outlined in this opinion. This includes the nature of the agent/food, for example an indispensable nutrient (i.e. selenium) and a situation where the risk is due to one component in a food whilst the benefit is due to another (i.e. fish).
Risk-benefit assessment of an indispensable nutrient: Selenium
Disclaimer: This example is not designed to provide conclusions as to risk-benefit of the specific food, but rather to highlight problem formulation and scoping of the risk-benefit assessment. The Scientific Committee has not reviewed the evidence of the selected health benefits and risks mentioned below.
Problem formulation
Selenium is an indispensable nutrient and is incorporated as selenocysteine into specific selenoproteins in both a dose- and tissue-dependent pattern, within a certain range of intake and under control of homeostatic mechanisms. Unspecific selenium incorporation of other selenised amino acids into body proteins is also possible, particularly when sulphur amino acids are deficient in the diet. Seleniumdependent glutathione peroxidases are part of the body’s defence system against oxidative stress. Selenium-dependent iodothyronine deiodinases regulate thyroid hormone metabolism. Chronic toxicity of selenium (selenosis) has been observed in humans with blood selenium concentrations > 100 μg/dL which correspond to a selenium intake above 850 μg/day and manifest as brittle hair and nails and hair loss, associated with gastrointestinal disturbances, skin rashes, garlic breath odour, fatigue, irritability and abnormalities of the nervous system (Yang et al. 1989). There are some indications that selenium intakes beyond amounts necessary to maximise selenoproteins in plasma reduce the risk of prostate, colon and total cancer (Clark et al., 1996; Yoshizawa et al., 1999) in the adult population. Infants of mothers with diets deficient in both iodine and selenium are at increased risk of congenital hypothyroidism (Vanderpas et al., 1992). Selenium deficiency may increase the virulence of certain enteroviruses for humans. In selenium depleted animals an amyocarditic strain of coxsackievirus B3 was converted to a virulent strain accompanied by changes in the genetic structure of the virus so that its genome closely resembled that of other known virulent CVB3 strains (Beck et al., 1995; 2003).
Example of problem formulation: “What is the balance between risks and benefits at the current levels of selenium intake in the population?”
Endpoints of relevance for the risk-benefit assessment
The risk and benefit relate in all groups of the population to insufficient, adequate or excessive intakes of selenium. Due to the limited evidence associated with the reduced risk of cancer, the focus of this example is on selenosis for the risk, and reduced risk of deficiency for the benefit.
| Type of effect | Endpoint | Target Population | Human health relationship |
|---|---|---|---|
| Risk | Selenosis | Whole population | Increased risk of selenosis at intakes above the Upper Level (UL) (EFSA, 2006a) |
| Benefit | Cancer | Adult population | Reduced risk of cancer (Clark et al, 1996; Yoshizawa et al., 1999) |
| Benefit | Normal levels of selenoenzymes and other selenoproteins | Whole population | No signs of deficiency, e.g. normal thyroid function at intakes above the Lower Threshold Intake (LTI) |
Risk-benefit assessment
Step 1 – Initial assessment
In the initial assessment, estimated dietary exposure to selenium of the population is compared to the health based guidance value (tolerable upper intake level, UL) and to the lower threshold intake (LTI). The LTI is by definition not a health based guidance value but is the lowest estimate of the requirement from the normal distribution curve (EFSA NDA Panel, 2010).
Scenario 1: Maximising the risks.
Identify a high level, e.g. 95th percentile, of current dietary exposure to selenium. Possible outcomes are:
a) The high level of dietary exposure to selenium is above the UL (and above the LTI),
Conclude that there is an appreciable risk of selenium toxicity and a benefit (i.e. no risk for selenium deficiency). Report to the risk-benefit manager that there is a risk for toxicity which could be reduced without affecting the benefit. Discuss Terms of Reference (II) aimed at identifying an appropriate dietary intake.
b) The high level of dietary exposure to selenium is below the LTI (and below the UL)
Conclude that there is no appreciable risk of selenium toxicity but there is an appreciable risk of selenium deficiency at this level of exposure. Report to the risk-benefit manager that the risks outweigh the benefits and make proposal to stop the assessment.
c) The high level of dietary exposure to selenium is below the UL and above the LTI
Conclude that there is no risk of selenium toxicity and a benefit (i.e. no risk for selenium deficiency) at this level of exposure. Report to the risk-benefit manager that the benefits outweigh the risks and make proposal to stop the assessment.
Scenario 2: minimising the risks.
Identify a low level, e.g. 5th percentile, of current selenium intake in the population. Possible outcomes are:
d) The low level of dietary exposure to selenium is above the UL (and above the LTI)
Conclude that there is an appreciable risk of selenium toxicity and a benefit (i.e. no risk for selenium deficiency). Report to the risk-benefit manager that there are clear risks and benefits at this level of exposure and discuss Terms of Reference (II) aimed at identifying an appropriate dietary intake.
e) The low level of dietary exposure to selenium is below the LTI (and below the UL).
Conclude that there is no appreciable risk of selenium toxicity but there is an appreciable risk of selenium deficiency at this level of exposure. Report to the risk-benefit manager that there is a risk of deficiency at current levels of exposure and discuss Terms of Reference (II) aimed at identifying an appropriate dietary intake.
f) The low level of dietary exposure to selenium is below the UL and above the LTI,
Conclude that there is no risk of selenium toxicity and there is a benefit (i.e. no risk for selenium deficiency) at this level of exposure. Risks at higher level of exposure will be determined by the outcome of scenario 1 (outcome “a” and “c”).
Step 2 – Refinement of the assessment
Following the discussion between the risk-benefit assessor and the risk-benefit manager on the outcome of step 1, refined Terms of Reference (II) are agreed upon, for example focussing on identifying suitable dietary intake levels at which it is possible to have the benefit of sufficiency without the risk of toxicity.
If suitable data are available, the exposure assessment could be refined - this could take the form of a probabilistic analysis of the dietary intake of selenium by the population. This would allow estimates of the proportions of the population with dietary exposure above the LTI and below the UL. Depending on the Terms of Reference (II), the analysis could be repeated with different dietary intake scenarios, which will give an indication of the increase or decrease in the risk and the benefit at specified dietary intake levels.
Depending on the Terms of Reference (II), if it is found that risks far outweigh the benefits or benefits far outweigh the risks at specified dietary intake levels, the report to the risk-benefit manager could conclude that the assessment could stop. If neither risks nor benefits prevail, then the advice to the risk-benefit manager could include consideration of whether or not, it is feasible to convert the health risk and benefit into a composite metric. If conversion is not possible, or theoretically possible but lacking in the necessary data, then identification of data needs would be helpful.
Step 3 – Comparison of risks and benefits using a composite metric
Following the discussion between the risk-benefit assessor and the risk-benefit manager on the outcome of step 2, refined Terms of Reference (III) are agreed upon, utilising a composite metric (e.g. DALY) and aiming at identifying a dietary intake level at which there is an agreed balance between the risk and the benefit. All of the data in this case study are based on human observations, which facilitates the application of a composite metric.
Risk-benefit assessment of fish consumption and exposure to methylmercury
Disclaimer: This example is not designed to provide conclusions as to risk-benefit of the specific food, but rather to highlight problem formulation and scoping of the risk-benefit assessment. The Scientific Committee has not reviewed the evidence of the selected health benefits and risks mentioned below.
Problem formulation
Consumption of fish is often recommended based on its nutritional benefits, but there is concern about a number of contaminants that can be present in different types of fish. Therefore formulation of advice to consumers requires definition of the amounts of fish that would be associated with the respective positive health effects and toxicological hazards. There have been a number of reviews of benefits and risks of fish consumption (e.g. SACN/COT, 2004; Becker et al., 2007; IoM, 2007; VKM, 2006; FDA, 2009, Cohen et al., 2005).
The beneficial components of fish include long-chain n3-polyunsaturated fatty acids (n3-LCPUFAs), a number of important vitamins and essential elements, and protein that is less associated with saturated animal fat than for example meat. The content of these nutrients varies in different fish species and varying amounts can also be provided by food sources other than fish. In principle a complete assessment would need to take into account the beneficial effects of increasing intake of these components and the adverse effects that could be associated with decreasing intake, taking into account other dietary sources of the nutrients and contaminants. This would make an assessment extremely complex and hence the approach has generally been to focus on the n3-LCPUFAs, for which fish is the major dietary source. Similarly there are many chemical contaminants present in fish. Persistent organic pollutants generally occur at highest levels in oily fish. Methylmercury is found predominantly in large predatory fish. Other types of contaminant may result from specific pollution incidents. It would not be feasible for a risk-benefit assessment to consider all potential contaminants in detail.
Example of problem formulation: “What is the balance between the benefits associated with the n3- LCPUFAs and the risks associated with methylmercury at current levels of fish consumption in the population?”
Endpoints of relevance for the risk-benefit assessment
The risks and benefits relate to different health effects, different types of fish and sometimes different population subgroups (see table below). Whilst a number of beneficial and adverse effects have been investigated, the strongest evidence is for protection by oily fish against a recurrence of myocardial infarction and for the risks of methylmercury, which is not necessarily associated with oily fish, with respect to neurodevelopmental effects.
| Type of effect | Endpoint | Target Population | Human health relationship |
|---|---|---|---|
| Risk | Motor and cognitive milestones of offspring | Women up to one year before and during pregnancy | Impaired neurodevelopment due to methylmercury |
| Risk | Motor and cognitive performance | Children | Impaired neurodevelopment due to post-natal dietary exposure to methylmercury |
| Risk | Coronary heart disease, Stroke | Adults | Increased risk of cardiovascular disease due to methylmercury |
| Benefit | Coronary heart disease, Stroke | Middle-aged and older people, especially those with previous myocardial infarction | Reduced risk of cardiovascular disease due to n3-LCPUFAs (proposed in reviews such as SACN/COT, 2004; Becker et al., 2007; IoM, 2007; VKM, 2006) |
| Benefit | Birth weight | Pregnant women | Reduced risk of low birth weight in (premature) infants due to n3-LCPUFAs |
| Benefit | Visual acuity of offspring | Pregnant women | Improved neurodevelopment due to n3-LCPUFAs |
| Benefit | Motor and cognitive milestones of offspring | Pregnant women | Improved neurodevelopment due to n3-LCPUFAs |
Risk-benefit assessment
Step 1 – Initial assessment
A number of approaches may be taken in the exposure assessment depending on the data that are available. For the purpose of this case study oily fish, which contain high levels of n3-PUFAs, and fish that contain relatively high levels of methylmercury, such as shark, swordfish and tuna, are considered separately. An alternative approach might be to use data for all fish combined, but this would introduce further uncertainty into the assessment.
Estimated intakes are compared to existing health-based guidance values, such as the Provisional Tolerable Weekly Intake (PTWI) for methylmercury of 1.6 μg/kg b.w. (FAO/WHO, 2007) and consumption of at least one portion of oily fish per week, in line with the recommendation of some authorities to obtain the positive health effects (SACN/COT, 2004; Becker et al., 2007).
Scenario 1: maximising the risks and minimising the benefits.
Identify a high level, e.g. 95th percentile, of dietary exposure to methylmercury from fish and a low level, e.g. 5th percentile, of consumption of oily fish. Possible outcomes are:
a) High level dietary exposure to methylmercury is below the PTWI and low level consumption of oily fish is at least one portion per week,
Conclude that there are no appreciable risks and there are clear benefits. Report to the risk-benefit manager that benefits far outweigh risks, and propose that the assessment can stop.
b) High level dietary exposure to methylmercury is below the PTWI and low level consumption is less than one portion of oily fish per week,
Conclude that there are no appreciable risks, and consider whether there are benefits under scenario 2 (outcome “e” or “f”). In the case of outcome “e”, report back to the risk-benefit manager that there is no appreciable risk but a possible benefit and propose to stop the risk-benefit assessment and continue with a benefit assessment. In the case of outcome “f”, report back to the risk-benefit manager that there are neither risks nor benefits and discuss Terms of Reference (II) aimed at identifying an appropriate dietary intake to try to optimise the benefits without inducing appreciable risks.
c) High level dietary exposure to methylmercury is above the PTWI. and low level consumption is at least one portion of oily fish per week,
Conclude that there are clear benefits and possible risks. Report to the risk-benefit manager and discuss Terms of Reference (II) to refine the risk-benefit assessment.
d) High level dietary exposure to methylmercury is above the PTWI and low level consumption is less than one portion of oily fish per week,
Conclude that there are possible risks and consider whether there are any benefits under scenario 2 (outcome “g” and “h”). In the case of outcome “g”, report back to the risk-benefit manager that there there are both risks and benefits and discuss Terms of Reference (II) aiming refining the risk-benefit assessment. In the case of outcome “h”, report back to the risk-benefit manager that there are clear risks and no discernable benefits. Propose to stop the risk-benefit assessment and continue the risk assessment.
Scenario 2: minimising the risks and maximising the benefits
Identify a low level, e.g. 5th percentile, of dietary exposure to methylmercury from fish and a high level, e.g. 95th percentile, of consumption of oily fish. Possible outcomes are:
e) Low level dietary exposure to methylmercury is below the PTWI and high level consumption of oily fish is at least one portion per week,
Conclude that there are possible benefits and consider whether there are some risks under Scenario 1 (outcome “b” or “c”). ”). In the case of outcome “b”, report back to the risk-benefit manager that there are possible benefits and no appreciable risks. Propose to stop the risk-benefit assessment and continue the benefit assessment. In the case of outcome “c”, report back to the risk-benefit manager that there are both risks and benefits and discuss Terms of Reference (II) aiming refining the risk-benefit assessment.
f) Low level dietary exposure to methylmercury is below the PTWI. and high level consumption of oily fish is less than one portion per week,
Conclude that there are no benefits. Report to the risk-benefit manager and propose to stop the riskbenefit assessment and continue the risk assessment.
g) Low level dietary exposure to methylmercury is above the PTWI and at high level consumption of oily fish is at least one portion per week,
Conclude that there are clear risks and possible benefits. Report to the risk-benefit manager and discuss Terms of Reference (II) aiming refining the risk-benefit assessment
h) Low level dietary exposure to methylmercury is above the PTWI and high level consumption of oily fish is less than one portion per week,
Conclude that there are clear risks and no discernable benefit. Report to the risk-benefit manager that risks far outweigh benefits, and propose that the assessment can stop.
Step 2 – Refinement of the assessment
Following the discussion between the risk-benefit assessor and the risk-benefit manager on the outcome of step 1, refined Terms of Reference (II) are agreed upon, for example focussing on particular subgroups or exposure scenarios.
If suitable data are available, the exposure assessments could be refined - this could take the form of a probabilistic analysis of the distributions of methylmercury occurrence in, and consumption of, different types of fish by the relevant population and subgroups. This would allow estimates of the proportions of the different subgroups, and of pregnant women, with dietary exposure above the PTWI or consuming less than one portion of oily fish per week. Depending on the Terms of Reference (II), the analysis (probabilistic or deterministic) could be repeated with different scenarios such as advice to consumers relating to amounts or types of fish to be consumed.
The available dose-response data can be modelled in order to estimate the likelihood (and in some instances magnitude) of the different hazards and positive effects at specified exposure levels, such as the mean, 5th percentile and 95th percentile of the relevant population groups. Applying this approach to the exposure modelled for different scenarios will give an indication of the increase or decrease in the risk and the benefit at specified dietary intake levels.
Finally, the above two approaches could be combined in an integrated probabilistic approach incorporating information on the individual variability in the identified health effects. In the above approaches the statistical uncertainty could be expressed in terms of confidence intervals, but the report to the risk-benefit manager should also describe the uncertainty with respect to the underlying data, e.g. if it is assumed that the dietary habits of pregnant women are similar to those of other women.
Depending on the Terms of Reference (II), if it is found that risks far outweigh benefits or benefits far outweigh risks for relevant subgroups, the report to the risk-benefit manager could conclude that the assessment could stop. If neither risks nor benefits prevail, then the advice to the risk-benefit manager could include consideration of whether or not, it is feasible to convert the health risks and benefits into a composite metric. If conversion is not possible, or theoretically possible but lacking in the necessary data, then identification of data needs would be helpful.
Step 3 – Comparison of risks and benefits using a composite metric
Following the discussion between the risk-benefit assessor and the risk-benefit manager on the outcome of step 2, refined Terms of Reference (III) are agreed upon, leading to an assessment of the risks and benefits utilising a composite metric. All of the data in this case study are based on human observations, which facilitates the application of a composite metric.
Conclusions and recommendations
The Scientific Committee concludes that benefit assessment should mirror the risk assessment paradigm by introducing four steps, i.e. positive health effect identification, positive health effect characterisation (dose response assessment), exposure assessment and benefit characterisation. The Scientific Committee notes that especially the positive health effect characterisation needs to be further developed.
The stepwise approach for risk-benefit assessment is considered by the Scientific Committee to be scientifically sound and efficient with respect to time and resources needed to reach a conclusion. By introducing a stepwise approach, a conclusion may already be reached after a qualitative or semi quantitative assessment, without the need to go to a full quantitative assessment, which is very demanding of data that are often not available. The examples provided highlight the complexity of risk-benefit assessment, already when entering the first steps of the assessment.
A full understanding between the risk-benefit assessor and the risk-benefit manager of the problem formulation and the resulting terms of reference is critical for ensuring a useful and relevant outcome for the risk-benefit management goals. After each step of the assessment, an iterative dialogue is foreseen between the risk-benefit assessor and the risk-benefit manager to eventually refine the terms of reference in view of the outcome of the previous step and the data available.
The Scientific Committee recommends that metrics used in risk-benefit assessment and weight factors associated to most common diseases should be internationally agreed upon in order to ensure harmonisation and recognition of the assessments.
The Scientific Committee recommends a close collaboration between risk assessors and benefit assessors in order to ensure that data generated by one or the other can be used in a broader riskbenefit assessment context. Further more, the development of hard biomarkers of effect for both risk and benefit is also needed.
Appendix
Common metrics for assessing separately risks and benefits
A number of metrics, suitable for use when assessing risks and benefits separately, are described in this appendix. See also IEA’s latest edition of Dictionary of Epidemiology, Editor Miquel Porta). There are three elements of health and disease impact, i.e. morbidity (frequency of disease), mortality (frequency of deaths) and disease burden (number of healthy days/years lost due to a disease). More than one metric will be needed to capture all three dimensions for use in a risk-benefit assessment.
A “quality of life metric”, measuring positive health effects is also needed for some risk-benefit assessments; unfortunately, generally agreed metrics for some positive health effects and well being are currently lacking, which may limit the benefit assessment to a qualitative characterisation of the positive health effect. A table presenting possible metrics to be used in risk-benefit assessment, with their advantages, disadvantages and data needs, is given below.
Composite metrics for comparing risks and benefits
The metrics commonly used for disease burden are disability adjusted life years (DALYs) and quality adjusted life years (QALYs). A discussion of these measures, and how they differ, is provided in Gold et al (2002).
DALY
WHO has developed the DALY metric as part of the effort to estimate global disease burden. The DALY includes morbidity, sequelae and mortality in one metric. For further information see the homepage http://www.who.int/healthinfo/global_burden_disease/en/index.html
DALYs for a disease or injury cause are calculated as the sum of the years of life lost due to premature mortality (YLL) in the population and the years lost due to disability (YLD) for incident cases of the disease or injury.
Therefore, DALY = YLL + wYLD.
YLL are calculated from the number of deaths at each age multiplied by a global standard life expectancy for each age. Thus:
YLL = (number of fatal cases) × (expected life span at the time of death)
YLD for a particular cause in a particular time period are estimated as follows:
YLD = (number of incident cases in that period) × (average duration of the disease).
“w” is the disability weight factor associated to the considered disease. The weight factor reflects the severity of the disease on a scale from 0 (perfect health) to 1 (death). WHO has set a list of different diseases and their respective weights. Weights can vary with population.
QALY
The QALY provides a composite metric for disease burden adjusted for the quality of life. Hence the metric is a complement to the DALY concept.
A QALY takes into account both the quantity and the quality of life generated by a given intervention, which may have a positive or negative effect on health. A QALY is the arithmetic product of life expectancy and the QALY valuation of the health state for the remaining life-years.
QALY = YLH + (1-w)YLD,
YLH is the number of years lived healthy.
The QALY metric is based on the number of years of life with perfect health that would be added due to a positive health effect. A year of perfect health is worth 1, while a year of less than perfect health, for example if the patient would be blind or confined to a wheelchair, is worth less than 1. Death is considered to be equivalent to 0, although, some health states may be considered worse than death and have negative scores. Again, weights may vary with the population.
Table 1: Overview of metrics that could be applied in risk-benefit assessment
| Metric | Description | Data needs |
|---|---|---|
| Mortality | Mortality risk, mortality rates, life expectancy (from birth), years of life lost (YLL) | Cause/age specific mortality
Risk-factor related mortality |
| Morbidity | Incidence of disease, morbidity risk | Prevalence and/or incidence data |
| Quality of life (QoL) | Consequences of morbidity and health impact not captured by disease, e.g. physical and mental health | Quality of Life indicators |
| DALY
(disability-adjusted life years) |
Combines information on severity and duration of a disease in terms of premature mortality and morbidity | Standard life-expectancy per age group, sex, country/region
Disease specific information on years of life lost due to premature mortality Disease incidence and specific information on years lived with disability Disease weights for severity |
| QALY
(quality-adjusted life years) |
Expected number of healthy years (number of years multiplied by the health-related quality of life during those years) | Disease incidence in a population
Duration of disease impact Health impact of disease |
| HALE
(healthy-life expectancy or health-adjusted life expectancy) |
Healthy life expectancy summarizes total life expectancy into
equivalent years of "full health" Taking into account years lived in less than full health due to diseases and injuries |
Period life-table (mortality rates by age and sex)
Prevalence of various states of health at different ages Time spent in non-optimal health state |
| ALE
(active life expectancy) |
Number of years an individual can expect to live without functional
limitations. Combines information on functional status and mortality Can assess expected life in a variety of functional states (without limitations, or with moderate or severe limitations) |
Expected years of life remaining per age group
Prevalence of functional limitations Person years lived in various stages of functioning |
| HLY
(healthy life years, disability free life expectancy) |
Number of years a person would be expected to live free of any
activity limitation |
Mortality statistics
Prevalence of diseases Health-related quality of life measures |
Abbreviations
| ADI | Acceptable Daily Intake |
| ARfD | Acute Reference Dose |
| BMDL | BenchMark Dose Lower confidence limit |
| DALYs | Disability Adjusted Life Years |
| LTI | Lower Threshold Intake |
| MOE | Margin Of Exposure |
| NOAEL | No-Observed-Adverse-Effect Level |
| n3-LCPUFAs | Long-Chain n3-PolyUnsaturated Fatty Acids |
| PBBD | Physiologically Based BioDynamic |
| PBBK | Physiologically Based BioKinetic |
| PCBs | PolyChlorinated Biphenyls |
| PTWI | Provisional Tolerable Weekly Intake |
| QALYs | Quality Adjusted Life Years |
| RBM | Risk-Benefit Manager |
| RCT | Randomised Controlled Trials |
| RDI | Recommended Daily Intake |
| TDI | Tolerable Daily Intake |
| UL | Upper Level |
Key words
Risk, benefit, assessment, problem formulation, risk-benefit assessment paradigm, stepwise approach, metrics
Associated files
Full 40 pages document:
File:EFSA Guidance for risk-benefit asssments July 2010.pdf
References
- ↑ [1]: EFSA Journal 2009; 7(9):1249 For citation purposes: EFSA Scientific Committee; Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, on request of EFSA. EFSA Journal 2009; 7(9):1249. [19 pp.]. doi:10.2093/j.efsa.2009.1249. Available online: www.efsa.europa.eu © European Food Safety Authority, 2009 1